
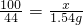 ��
��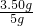 ��100%=70.00%��
��100%=70.00%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������н϶�ơ�þ���ӵ�ˮ���������ʯ��֢�����ȿ�ʹˮ�иơ�þ����ת��Ϊ����—ˮ����������Ҫ�ɷ���CaCO3��Mg(OH)2��Ϊȷ��ˮ���ijɷ֣����ʵ��С�����������ʵ�飺
�ٳ�ȡ5gˮ����Ʒ��������100mL�ձ��У�Ȼ��ע��50 g 10%��ϡ���
�ڷ�Ӧֹͣ��Ӧ����������������1.54g��
����ȡ����ˮ������ʵ��ڵĻ��Һ�У������ݲ�����
�����������Ϣ�ش�
��1��ʵ��۵�Ŀ���� ��
��2������ˮ����Ʒ��̼��Ƶ�����������Ҫ����ݻ�ѧ����ʽ���㣬��������ȷ��0.01����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ȫ���п�����ר����ר������ѧ����ʽ���㣨���� ���ͣ�������
��10����2-8����4�֣������������н϶�ơ�þ���ӵ�ˮ���������ʯ��֢�����ȿ�ʹˮ�иơ�þ����ת��Ϊ��������ˮ����������Ҫ�ɷ���CaC03��Mg(OH)2��Ϊȷ��ˮ���ijɷ֣����ʵ��С�����������ʵ�飺
�ٳ�ȡ5gˮ����Ʒ��������lOOmL�ձ��У�Ȼ��ע��50gl0%��ϡ���
�ڷ�Ӧֹͣ��Ӧ����������������1. 54g;
����ȡ����ˮ������ʵ��ڵĻ��Һ�У������ݲ�����
�����������Ϣ�ش�
(l)ʵ��۵�Ŀ����________��
(2)����ˮ����Ʒ��̼��Ƶ���������(Ҫ����ݻ�ѧ����ʽ���㣬��������ȷ��0.01)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����мö���ѧ���꼶���£��¿���ѧ�Ծ���5�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com