
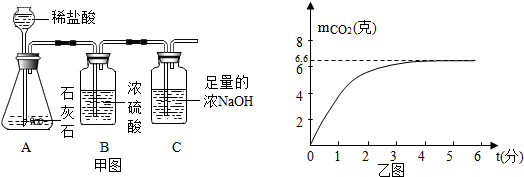
���� ��1�����ݶ�����̼�������ͻ�ѧ����ʽ����̼��Ƶ�����������������������
��2������AB�����ڻ���ʣ��Ķ�����̼û����ȫ�������������ս��з�����
��� �⣺��1����̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 6.6g
$\frac{100}{44}=\frac{x}{6.6g}$
x=15g
��ʯ��ʯ��CaCO3����������Ϊ��$\frac{15g}{30g}$��100%=50%��
��2��AB�����ڿ��ܻ���ʣ��Ķ�����̼û����ȫ�������������գ�����Cװ�ò�õĶ�����̼�����뷴Ӧ�����Ķ�����̼��������ȣ�
�ʴ�Ϊ����1����ʯ��ʯ��CaCO3����������Ϊ50%��
��2��AB�����ڻ���ʣ��Ķ�����̼û����ȫ�������������գ��ӳ���©�����뵪������������̼ȫ���ų���
���� �����ѶȽϴ���ȷʵ�����ԭ���������ͼ������ȷ������⣮


 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
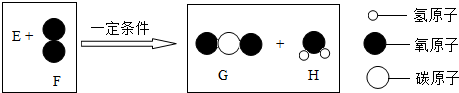
| A�� | H����Ԫ�ص�����Ϊ2�� | B�� | EΪ���������� | ||
| C�� | G��H�ķ��Ӹ�����Ϊ1��2 | D�� | E������ԭ�Ӹ�����Ϊ1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaCl��Na2CO3�� | B�� | C �ۣ�CuO�� | C�� | KNO3��KCl�� | D�� | Cu �ۣ�Fe�ۣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Һ | B�� | ��ˮ����� | C�� | O2��O3�Ļ���� | D�� | �ӵ�ʳ�ξ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com