
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| �ܽ��/g | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | 60.2 | 65.6 |
| 2000g��3% |
| 2000g-500g |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?������һģ��ij��ѧС��ͬѧ������ͼ��ʾװ�ý���ʵ�飮
��2013?������һģ��ij��ѧС��ͬѧ������ͼ��ʾװ�ý���ʵ�飮
| ||
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?������һģ��ͬѧ��������������������ij��Ԫ�صĻ��ϼ۹����˳��л�ѧ�������ʼ��ת����ϵ����ͼ��ͼ�С�������ʾ���ʼ��ת������
��2013?������һģ��ͬѧ��������������������ij��Ԫ�صĻ��ϼ۹����˳��л�ѧ�������ʼ��ת����ϵ����ͼ��ͼ�С�������ʾ���ʼ��ת�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
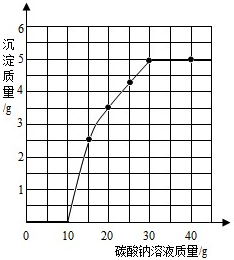 ��2013?������һģ��ʵ�����ù�����ϡ����ʹ���ʯ��ȡCO2��ȡ50g��Ӧ�����Һ����ε���̼������Һ����õ���̼������Һ�����������������������ϵ��ͼ��ʾ��
��2013?������һģ��ʵ�����ù�����ϡ����ʹ���ʯ��ȡCO2��ȡ50g��Ӧ�����Һ����ε���̼������Һ����õ���̼������Һ�����������������������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com