Ė®¶ŌĪŅĆĒµÄÉś²śŗĶÉś»ī¶¼ŹĒ·Ē³£ÖŲŅŖµÄ£®
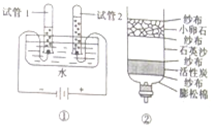
£Ø1£©ČēĶ¼¢ŁĖłŹ¾ŹĒµē½āĖ®µÄŹµŃ飬Š“³öÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½
£¬
ĘäÖŠŹŌ¹Ü
2
2
£ØĢī”°1”±»ņ”°2”±£©ÖŠŹÕ¼Æµ½µÄĘųĢåŹĒŃõĘų£®
£Ø2£©Ä³Ķ¬Ń§ŅŖ¾»»ÆŹÕ¼Æµ½µÄÓźĖ®£¬×ŌÖĘĮĖŅ»øö¼ņŅ×¾»Ė®Ę÷£ØČēĶ¼¢Ś£©£¬ĘäÖŠŠ”ĀŃŹÆ”¢ŹÆӢɳŗĶÅņĖÉĆŽµÄ×÷ÓĆŹĒ
¹żĀĖ
¹żĀĖ
£®
£Ø3£©Ź¢×°æóČŖĖ®ÓƵÄĖÜĮĻĘæĒį±ćĪĄÉś£¬æÉŅŌ»ŲŹÕŌŁĄūÓĆ£¬ĘäĖłÓĆ²ÄĮĻŹōÓŚ
B
B
£ØĢīŠņŗÅ£©
A£®ø“ŗĻ²ÄĮĻ B£®ÓŠ»śøß·Ö×Ó²ÄĮĻ
C£®¹čĖįŃĪ²ÄĮĻ D£®½šŹō²ÄĮĻ
£Ø4£©ĪŅĆĒÓ¦µ±ÕäĻ§ĆæŅ»µĪĖ®£¬ĻĀĮŠ×ö·Ø²»ĄūÓŚ½ŚŌ¼ÓĆĖ®µÄŹĒ
C
C
£ØĢī±źŗÅ£©£®
A£®Ļ“²ĖµÄĖ®ÓĆĄ“½½»Ø B£®Ź¹ÓĆ½ŚĖ®ĮśĶ· C£®ÓĆ²»¼ä¶ĻµÄĖ®Į÷³åĻ“Ķėæź D£®Ļ“ŹÖ²Į·ŹŌķŹ±£¬¹ŲÉĻĖ®ĮśĶ·
£Ø5£©Éś»īÖŠ½µµĶĖ®µÄÓ²¶Č³£ÓƵķ½·ØŹĒ
¼ÓČČÖó·Š
¼ÓČČÖó·Š
£®

 Ė®¶ŌĪŅĆĒµÄÉś²śŗĶÉś»ī¶¼ŹĒ·Ē³£ÖŲŅŖµÄ£®
Ė®¶ŌĪŅĆĒµÄÉś²śŗĶÉś»ī¶¼ŹĒ·Ē³£ÖŲŅŖµÄ£®

 ĢĘÓ”ĪÄ»ÆæĪŹ±²āĘĄĻµĮŠ“š°ø
ĢĘÓ”ĪÄ»ÆæĪŹ±²āĘĄĻµĮŠ“š°ø µ¼Ń§Óė²āŹŌĻµĮŠ“š°ø
µ¼Ń§Óė²āŹŌĻµĮŠ“š°ø
 Ė®¶ŌĪŅĆĒµÄÉś²śŗĶÉś»ī¶¼ŹĒ·Ē³£ÖŲŅŖµÄ£®
Ė®¶ŌĪŅĆĒµÄÉś²śŗĶÉś»ī¶¼ŹĒ·Ē³£ÖŲŅŖµÄ£® Ė®¶ŌĪŅĆĒµÄÉś²śŗĶÉś»ī¶¼ŹĒ·Ē³£ÖŲŅŖµÄ£®
Ė®¶ŌĪŅĆĒµÄÉś²śŗĶÉś»ī¶¼ŹĒ·Ē³£ÖŲŅŖµÄ£®
