�⣺��1���Ҽ���ˮ����ʹ�û���̿��ˮ���������˻���̿�������ԣ�
��2��ϴ�ྫ�����黯���ܣ����Բ;��ϵ������ü���ϴ�ྫ��ˮ������ϴ��
��3��ԭ�ӽṹʾ��ͼ��֪����������������������Ӳ����ȣ��Ȼ�������Ԫ�ص�ԭ�ӽṹʾ��ͼ��֪��ԭ�����������Ӳ㣻
��4��ȼ�յ�������֪��ȼ��ȼ�ղ�����Ҫ����������ͬʱ�߱��¶ȴﵽ�Ż�㣻
��5��һ����̼���л�ԭ�ԣ�һ����̼���Ի�ԭ�������������Ͷ�����̼����ѧ����Ϊ��3CO+Fe
2O
3
3CO
2+2Fe���������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0��Fe
2O
3����Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����Ԫ�صĻ��ϼ�Ϊ+3�ۣ���Ԫ�ص���������=
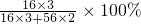
=30%��
�ʴ�Ϊ����1����������2���黯����3����ԭ�����������Ӳ㣻��4���¶ȴﵽ�Ż�㣻��5��3CO+Fe
2O
3
3CO
2+2Fe����ԭ��+3�ۣ�30%��
��������1�����ݻ���̿���������Խ��н��
��2������ϴ�ྫ�����黯���ܽ��н��
��3������ԭ�ӽṹʾ��ͼ��֪����������������������Ӳ����Ƚ��н��
��4������ȼ�յ�������֪��ȼ��ȼ�ղ�����Ҫ����������ͬʱ�߱��¶ȴﵽ�Ż����н��
��5������һ����̼���л�ԭ�Կ��Ի�ԭ�������������Ͷ�����̼�Լ��������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0���н��
�����������㿼�������ʵ���������;������Ҳ�����ˣ��������ʾ�����;����;�ַ�ӳ���ʵ���������㾭��������ѡ�����������У�

 ��ѧ�ܸ������ǵ����������������������ƶ����Ľ������������ѧ�Ļ�ѧ֪ʶ�ش��������⣮
��ѧ�ܸ������ǵ����������������������ƶ����Ľ������������ѧ�Ļ�ѧ֪ʶ�ش��������⣮ 3CO2+2Fe���������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0��Fe2O3����Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����Ԫ�صĻ��ϼ�Ϊ+3�ۣ���Ԫ�ص���������=
3CO2+2Fe���������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0��Fe2O3����Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����Ԫ�صĻ��ϼ�Ϊ+3�ۣ���Ԫ�ص���������=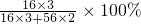 =30%��
=30%�� 3CO2+2Fe����ԭ��+3�ۣ�30%��
3CO2+2Fe����ԭ��+3�ۣ�30%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 ��2012?��������ѧ�ܸ������ǵ����������������������ƶ����Ľ������������ѧ�Ļ�ѧ֪ʶ�ش��������⣮
��2012?��������ѧ�ܸ������ǵ����������������������ƶ����Ľ������������ѧ�Ļ�ѧ֪ʶ�ش��������⣮ ��ѧ�ܸ������ǵ����������������������ƶ����Ľ������������ѧ�Ļ�ѧ֪ʶ�ش��������⣮
��ѧ�ܸ������ǵ����������������������ƶ����Ľ������������ѧ�Ļ�ѧ֪ʶ�ش��������⣮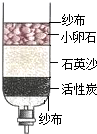 ��ѧ�ܸ������ǵ����������������������ƶ����Ľ������������ѧ�Ļ�ѧ֪ʶ�ش��������⣮
��ѧ�ܸ������ǵ����������������������ƶ����Ľ������������ѧ�Ļ�ѧ֪ʶ�ش��������⣮ Pb+2H2SO4+______��
Pb+2H2SO4+______��