
| ��Ӧʱ�� | T0 | T1 | T2 | T3 |
| �ձ���ҩƷ����/g | 151.4 | 151.3 | 151.0 | 151.0 |
| ��̼�� | ��̼�� | ��̼�� |
| ��̼��0.03%-0.3% | ��̼��0.3%-0.6% | ��̼��0.6%-2% |
| 56 |
| 2 |
| x |
| 0.4g |
| 11.4g-11.2g |
| 11.4g |


 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijУ��ѧ������ȤС���ͬѧ���о���ѧϰ����չʾ��һ������ͼ��ʾʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ��μӷ�Ӧ�����������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��H2C2O4
ijУ��ѧ������ȤС���ͬѧ���о���ѧϰ����չʾ��һ������ͼ��ʾʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ��μӷ�Ӧ�����������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��H2C2O4| Ũ���� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 CO��+CO2��+H2O
CO��+CO2��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
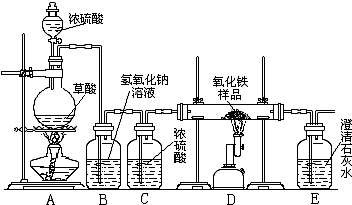
ijУ��ѧ������ȤС���ͬѧ���о���ѧϰ����չʾ��һ������ͼ��ʾ��ʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ��μӷ�Ӧ����

�������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��
![]() H2C2O4 CO��+CO2+H2O
H2C2O4 CO��+CO2+H2O
ͨ�����ۣ�ͬѧ�Ƕ�����װ�����˶�����ʶ��
��1����һС��ͬѧ˵����ʵ�鰲ȫ��ʵ���������,����Ҫ���װ�õ�������,ʵ�鿪ʼ�ȼ���___����A��D������ʵ�����ʱ��Ӧ_____�����Ȼ��ֹͣD���ļ��ȣ��ӻ����ĽǶȽ�����Eװ�ú�Ӧ��β�����д������䷽���ǣ� ��
��2���ڶ�С���ͬѧ˵���ø�ʵ��װ�ÿ��Լ���һ����̼����������Ӧ�IJ������Bװ�õ�����_______ ��Eװ�õ�����___ ��
һ����̼����������Ӧ�Ļ�ѧ����ʽΪ ___________________ _ _��
��3������С���ͬѧ˵����������װ�û����Բⶨ��������Ʒ�����������������������ǵIJⶨ�����ǣ�������������Ʒ������10.0 g����Ʒ�벣���ܵ�������Ϊ60.0 g,��ȫ��Ӧ����ȴ���ٳ�����������ʣ������������Ϊ57.6 g������ʵ������������Ʒ������������������Ϊ ��
��4������С��ͬѧ˵����������װ�û�������ⶨ��Ʒ�������������������ķ������ȳ�����������Ʒ������,�ٷֱ����Eװ���ڷ�Ӧǰ��������������ɼ��������Ʒ�������������������������˷���ʵ��ʵ��ⶨ���ȴƫ�������ƫ���ԭ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com