
���� ��1���ٽ���ȼ��ȼ�յķ����У������ȼ���������ĽӴ����������������Ũ�ȣ��ݴ˽��з������
��2��������������ЧӦ�������ԭ���з������
��3������̼������ָ����������Ϣʱ�����õ�����Ҫ�������٣��ر��Ǽ��ٶ�����̼���ŷ�����������̬�����Դӽڵ硢���ܺͻ��յȻ������ı�����ϸ�ڣ��ݴ˽��з�����ɣ�
��� �⣺��1��ú��ȼ��ʱ����ú������ú����Ϊ��ʹú�������ֽӴ�������ú���ȼ�գ�
��2���ܶ����������г�����Ч�ؼ����˶�����̼����������һ����̼��������ŷţ���Щ�����л���������ЧӦ���Ƕ�����̼�����������Ƕ�������
��3��A������һ���Ե�ľ�꣬�ܽ�Լ��ֽ���õ�ľ�ģ�������ľ�Ŀ�������ǿ������̼�����ģ���ѡ����ϡ���̼������
B�����������ε���ͷ���ܽ�Լ���ܣ��ܼ��ٶ�����̼���ŷţ���ѡ����ϡ���̼������
C��������չ�������磬����������Ķ�����̼����ѡ����ϡ���̼������
�ʴ�Ϊ����1����ֽӴ�����2��������̼����������3��AB��
���� �����ѶȲ������մٽ���ȼ��ȼ�յķ���������ЧӦ�����ꡢ��̼�������������ȷ�����Ĺؼ���


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢܢݢ� | B�� | �ܢ٢ۢڢݢ� | C�� | �٢ۢڢܢݢ� | D�� | �٢ܢڢۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Һ����Һ�ڻ�ѧ�о���ʵ������������Ҫ���ã�
��Һ����Һ�ڻ�ѧ�о���ʵ������������Ҫ���ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
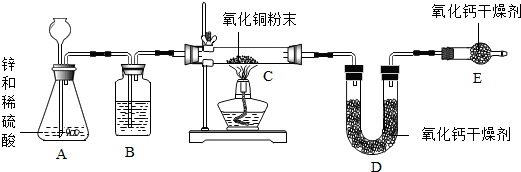
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ��a��b��c�������ʵ��ܽ�����ߣ�����ͼ�ش�
��ͼ��a��b��c�������ʵ��ܽ�����ߣ�����ͼ�ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ����������� | ����ķ��� | |
| A | ��ë����� | ȼ�գ�����ζ |
| B | �ռ�������� | ��ˮ������Һ����¶ȱ仯 |
| C | �������Ͻ� | ��̻� |
| D | �Ȼ��������� | ����ʯ�ң���ĥ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���� | K2CO3 | K2SO4 | KMnO4 |
| �ܽ��/g | 111 | 11.1 | 6.34 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
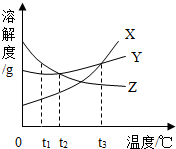 ��ͼ��X��Y��Z���ֹ������ʵ��ܽ������ͼ����������ͼ�ش��������⣮
��ͼ��X��Y��Z���ֹ������ʵ��ܽ������ͼ����������ͼ�ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯī�ͽ��ʯ������̼�ĵ��� | |
| B�� | CO2��ʹ�������ɫʯ��ֽ����� | |
| C�� | CO ���CO2�� | |
| D�� | ľ̿��CO ��һ�������¶���������ͭ��Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com