
| ϡ��������� | ʣ��������� |
| ��һ�μ���10g | 8g |
| �ڶ��μ���10g | 6g |
| �������10g | 4g |
| ���Ĵμ���10g | 3g |
| ������10g | 3g |
���� ��1�����õ�һ�κ͵�����ʣ�����������ɼ������Ʒ��̼��Ƶ�������
��2�����������ͼ��������ÿ10g�������ĵ�̼��Ƶ�����Ϊ2g���ɻ�ѧ����ʽ�ɵõ������ʵ������ȣ��г�����ʽ�����10g�����к������ʵ��������Ӷ��������ϡ�������������������
��3������ϡ��ǰ�����ʵ��������������
��� �⣺
��1������ͼ���е����ݿ�֪����Ʒ��̼��Ƶ�������10g-3g=7g��
��2�������֪��ÿ10g�������ĵ�̼��Ƶ�����Ϊ2g����10g�����к�����������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73
2g x
$\frac{100}{2g}=\frac{73}{x}$
x=1.46g
�����������������$\frac{1.46g}{10g}$��100%=14.6%
�����õ�ϡ���������ʵ�����������14.6%��
��3������Ҫ��ϡ��������Ϊy
14.6%��y=5%��500g
y=171.2g
ˮ������Ϊ500g-171.2g=328.7g
�ʴ�Ϊ��
��1��7��
��2����ϡ�������������������14.6%��
��3��171.2g��328.7g��
���� ���б���ļ����У�����Ҫ���ݱ��������������ݷ������ó���������Ҫ�����ʵ�������Ȼ�������û�ѧ����ʽ���м��㣮


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
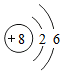 ��������ԭ�ӽṹʾ��ͼ��֪����ԭ���ڻ�ѧ��Ӧ���õ���ѡ��õ�����ʧȥ�������Ӵﵽ�ȶ��ṹ��
��������ԭ�ӽṹʾ��ͼ��֪����ԭ���ڻ�ѧ��Ӧ���õ���ѡ��õ�����ʧȥ�������Ӵﵽ�ȶ��ṹ��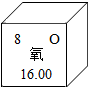 ��ʾ����ͼ����ֱ�ӻ�õ���Ϣ��AD��
��ʾ����ͼ����ֱ�ӻ�õ���Ϣ��AD��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
 ��100g���������ֱ�Ϊ3.65%�����12%MgSO4�Ļ����Һ�У��μ���������Ϊ3.42%��Ba��OH��2��Һ����������Ϊ���ڼ�����Ba��OH��2��Һ���ܶ�Ϊ1g/cm3��
��100g���������ֱ�Ϊ3.65%�����12%MgSO4�Ļ����Һ�У��μ���������Ϊ3.42%��Ba��OH��2��Һ����������Ϊ���ڼ�����Ba��OH��2��Һ���ܶ�Ϊ1g/cm3��| Ba��OH��2��Һ�����V��/mL | ||||
| �����Ļ�ѧʽ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ֱ����ȼ�ŵ�ľ�����۲����� | |
| B�� | �ֱ�ͨ��̼������Һ�У��۲����� | |
| C�� | �ֱ�ͨ����ɫʯ����Һ�У��۲����� | |
| D�� | �ֱ�ͨ�����ȵ�����ͭ���۲����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���ֵ����� | ����������ԭ���� |
| A | ����ָ������ָ | �ٹ۲���ɫ���ڵμ�ϡ����۲����� |
| B | Ũ�����ϡ���� | �ٴ�ƿ�ڹ۲졡 ������ˮ�����ձ� |
| C | ������������������ | �ٷֱ��ˮ�������ձ����ڷſ�����һ��ʱ��۲� |
| D | ʳ�κͰ��� | �ٳ�ζ������ �ڵμ���������Һ�۲� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10% | B�� | 20% | C�� | 25% | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �¶ȣ��棩 | 20 | 100 | 290 | 500 | 1000 |
| ʣ�����������g�� | 93.0 | 93.0 | 83.0 | 80.3 | 75.0 |
| A�� | 100��290�棺CoO��Co2O3 | B�� | 290�棺CoO | ||
| C�� | 500�棺Co3O4 | D�� | 1000�棺Co2O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ����� | a | b | c | d |
| ��Ӧǰ������g�� | 6.4 | 3.2 | 4.0 | 0.5 |
| ��Ӧ��������g�� | ���� | 2.56 | 7.2 | 0.5 |
| A�� | a��b�Ƿ�Ӧ�d�����Ǵ��� | |
| B�� | ��Ӧ��a���ʵ�����Ϊ3.84g | |
| C�� | c������Ԫ�ص����࣬һ������a��b����������Ԫ�ص����� | |
| D�� | ������a��b����Է�������֮��Ϊ2��1����Ӧ��a��b�Ļ�ѧ������֮��Ϊ1��2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com