
| ʵŃé | 1 | 2 | 3 | 4 |
| ĽÓČëŃůĆ·µÄÖĘÁż/g | 5 | 10 | 15 | 20 |
| ÉúłÉCO2µÄÖĘÁż/g | 1.54 | 3.08 | 4.4 | m |
| 100 |
| 5gˇÁx |
| 44 |
| 1.54g |
| 100 |
| y |
| 44 |
| 4.4g |
| 111 |
| z |
| 11.1g |
| 10g+50g-4.4g |


 ·˘É˘ËĽÎ¬ĐÂżÎĚĂϵÁĐ´đ°¸
·˘É˘ËĽÎ¬ĐÂżÎĚĂϵÁĐ´đ°¸
| Ä꼶 | ¸ßÖĐżÎłĚ | Ä꼶 | łőÖĐżÎłĚ |
| ¸ßŇ» | ¸ßŇ»Ăâ·ŃżÎłĚÍĆĽöŁˇ | łőŇ» | łőŇ»Ăâ·ŃżÎłĚÍĆĽöŁˇ |
| ¸ß¶ţ | ¸ß¶ţĂâ·ŃżÎłĚÍĆĽöŁˇ | łő¶ţ | łő¶ţĂâ·ŃżÎłĚÍĆĽöŁˇ |
| ¸ßČý | ¸ßČýĂâ·ŃżÎłĚÍĆĽöŁˇ | łőČý | łőČýĂâ·ŃżÎłĚÍĆĽöŁˇ |
żĆÄżŁşłőÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁş
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁşłőÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁş
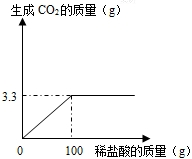 ÄłżÎÍâ»î¶ŻĐˇ×éÎŞ˛â¶¨µ±µŘĘŻ»ŇĘŻÖĐş¬ĚĽËá¸ĆµÄÖĘÁż·ÖĘýŁ¬ČˇŔ´ÁËһЩżóĘŻŁ¬×ĽČ·łĆȡŃůĆ·10g·ĹÓÚÉŐ±ÖĐŁ¨ÔÓÖʼȲ»ČÜÓÚË®Ł¬Ň˛˛»ÓëϡŃÎËá·´Ó¦Ł¬Ň˛˛»·Ö˝âŁ©Ł¬ĎňĆäÖĐĽÓČë×ăÁżµÄϡŃÎËᣬ¸ůľÝʵŃé˛âµĂµÄĘýľÝ»ćÖĆĎÂÍĽŁ®
ÄłżÎÍâ»î¶ŻĐˇ×éÎŞ˛â¶¨µ±µŘĘŻ»ŇĘŻÖĐş¬ĚĽËá¸ĆµÄÖĘÁż·ÖĘýŁ¬ČˇŔ´ÁËһЩżóĘŻŁ¬×ĽČ·łĆȡŃůĆ·10g·ĹÓÚÉŐ±ÖĐŁ¨ÔÓÖʼȲ»ČÜÓÚË®Ł¬Ň˛˛»ÓëϡŃÎËá·´Ó¦Ł¬Ň˛˛»·Ö˝âŁ©Ł¬ĎňĆäÖĐĽÓČë×ăÁżµÄϡŃÎËᣬ¸ůľÝʵŃé˛âµĂµÄĘýľÝ»ćÖĆĎÂÍĽŁ®˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁşłőÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁş
| ʵŃé | 1 | 2 | 3 | 4 |
| ĽÓČëŃůĆ·µÄÖĘÁż/g | 5 | 10 | 15 | 20 |
| ÉúłÉCO2µÄÖĘÁż/g | 1.54 | 3.08 | 4.4 | m |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁşłőÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁş
| c-b |
| a |
| c-b |
| a |
˛éż´´đ°¸şÍ˝âÎö>>
°Ů¶ČÖÂĐĹ - Á·Ď°˛áÁбí - ĘÔĚâÁбí
şţ±±Ęˇ»ĄÁŞÍřÎĄ·¨şÍ˛»ÁĽĐĹϢľŮ±¨Ć˝Ě¨ | ÍřÉĎÓĐş¦ĐĹϢľŮ±¨×¨Çř | µçĐĹթƾٱ¨×¨Çř | ÉćŔúĘ·ĐéÎŢÖ÷ŇĺÓĐş¦ĐĹϢľŮ±¨×¨Çř | ÉćĆóÇÖȨľŮ±¨×¨Çř
ÎĄ·¨şÍ˛»ÁĽĐĹϢľŮ±¨µç»°Łş027-86699610 ľŮ±¨ÓĘĎ䣺58377363@163.com