
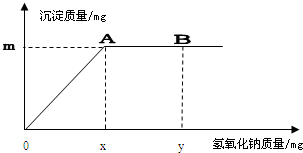
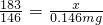 ���x=0.183mg�����ݣ�
���x=0.183mg�����ݣ�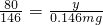 ���y=0.08mg
���y=0.08mg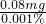 =80000mg��
=80000mg��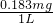 =0.183mg/L
=0.183mg/L

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2012��1�·�15����������������������Ⱦ�¼���������ע����Ⱦ�Ӷγ���30���������в������Ⱥ�ڵ���ˮ��ȫ����һ�ִ��Բⶨ��ˮ���Ӻ����ķ�����������ˮ�е�Cd2+ȫ����CdCl2���ڣ����䷴Ӧԭ���ǣ�CdCl2+2NaOH=Cd��OH��2��+2NaCl����ȡ�Ӷ�1000mLˮ���������м���һ�������ռ���Һ����ַ�Ӧ����ˣ�ϴ�ӣ�������������Ϊ0.146mg���Իش�
2012��1�·�15����������������������Ⱦ�¼���������ע����Ⱦ�Ӷγ���30���������в������Ⱥ�ڵ���ˮ��ȫ����һ�ִ��Բⶨ��ˮ���Ӻ����ķ�����������ˮ�е�Cd2+ȫ����CdCl2���ڣ����䷴Ӧԭ���ǣ�CdCl2+2NaOH=Cd��OH��2��+2NaCl����ȡ�Ӷ�1000mLˮ���������м���һ�������ռ���Һ����ַ�Ӧ����ˣ�ϴ�ӣ�������������Ϊ0.146mg���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ�����л�´����ѧУ�п������Ի�ѧ�Ծ����������� ���ͣ�������
��6�֣�2012��1�·�15����������������������Ⱦ�¼���������ע����Ⱦ�Ӷγ���30���������в������Ⱥ�ڵ���ˮ��ȫ����һ�ִ��Բⶨ��ˮ���Ӻ����ķ�����������ˮ�е�Cd2+ȫ����CdCl2���ڣ����䷴Ӧԭ���ǣ�CdCl2+2NaOH=Cd(OH)2��+2NaCl����ȡ�Ӷ�1000mLˮ���������м���һ�������ռ���Һ����ַ�Ӧ����ˣ�ϴ�ӣ�������������Ϊ0.146mg���Իش�
��1�������ⶨ��Ӧ�Ļ�������Ϊ ��
��2������˵���úӶ�ˮ���Ȼ��Ӻ����Ƿꡣ�����ұ�GB5749-2006����������ˮ��������CdCl2��0.008mg/L��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com