
̿����ͼ����һ�ּ��������������Ϊһ��IJ�Ʒ��
 ���ֲ�Ʒ�ɶԳ��ڼ����ڿ����е�һ����̼����ȩ���к�����
���ֲ�Ʒ�ɶԳ��ڼ����ڿ����е�һ����̼����ȩ���к�����
������Ч������ij����С�����̿���Ʒ���г���̽����
[�������]̿���Ʒ���Ƿ���̼Ԫ�ء�
[���������]̿���Ʒ�к���̼Ԫ��
[��������]��̼�ڿ�������ȫȼ��ֻ���ɶ�����̼������ȫȼ��
���ɶ�����̼��һ����̼��
�����ʵ��˻���ѪҺ����һ����̼���ʺ��Ϊ���졣
 [���ʵ��]ͨ������̿���Ʒȼ�ղ��֤��̿���Ʒ�к���̼Ԫ�ء���װ������������ͼ��
[���ʵ��]ͨ������̿���Ʒȼ�ղ��֤��̿���Ʒ�к���̼Ԫ�ء���װ������������ͼ��
��1�� Aװ�õ�����Ϊ���տ����е�ˮ�Ͷ�����̼��������������ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ�鿪ʼ��Cװ�����а�ɫ������Cװ�÷�����ѧ��Ӧ����ʽΪ ��Eװ�������ʵļ�Ѫ��Ϊ����ɫ����֤�� ��
[����������]��3��С����Ϊ������A��Bװ�ü䣬���ӳ���ʯ��ˮ��Ŀ���� ��
 ��4��С����Ϊ��������ͼ��ʾ��װ�ã�
��4��С����Ϊ��������ͼ��ʾ��װ�ã�
�滻ԭװ���е�Eװ�ã�����Ϊ��
������Ҫԭ�� ��
[��������]̿���Ʒ�к���̼Ԫ�ء�
[����̽��]�����̿���Ʒ����һ��̽�������ݣ���������Ľ��� ��
 �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̿����ͼ����һ�ּ��������������Ϊһ��IJ�Ʒ��
 ���ֲ�Ʒ�ɶԳ��ڼ����ڿ����е�һ����̼����ȩ���к�����
���ֲ�Ʒ�ɶԳ��ڼ����ڿ����е�һ����̼����ȩ���к�����
������Ч������ij����С�����̿���Ʒ���г���̽����
[�������]̿���Ʒ���Ƿ���̼Ԫ�ء�
[���������]̿���Ʒ�к���̼Ԫ��
[��������]��̼�ڿ�������ȫȼ��ֻ���ɶ�����̼������ȫȼ��
���ɶ�����̼��һ����̼��
�����ʵ��˻���ѪҺ����һ����̼���ʺ��Ϊ���졣
 [���ʵ��]ͨ������̿���Ʒȼ�ղ��֤��̿���Ʒ�к���̼Ԫ�ء���װ������������ͼ��
[���ʵ��]ͨ������̿���Ʒȼ�ղ��֤��̿���Ʒ�к���̼Ԫ�ء���װ������������ͼ��
��1�� Aװ�õ�����Ϊ���տ����е�ˮ�Ͷ�����̼��������������ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ�鿪ʼ��Cװ�����а�ɫ������Cװ�÷�����ѧ��Ӧ����ʽΪ ��Eװ�������ʵļ�Ѫ��Ϊ����ɫ����֤�� ��
[����������]��3��С����Ϊ������A��Bװ�ü䣬���ӳ���ʯ��ˮ��Ŀ���� ��
 ��4��С����Ϊ��������ͼ��ʾ��װ�ã�
��4��С����Ϊ��������ͼ��ʾ��װ�ã�
�滻ԭװ���е�Eװ�ã�����Ϊ��
������Ҫԭ�� ��
[��������]̿���Ʒ�к���̼Ԫ�ء�
[����̽��]�����̿���Ʒ����һ��̽�������ݣ���������Ľ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ֲ�Ʒ�ɶԳ��ڼ����ڿ����е�һ����̼����ȩ���к�����
���ֲ�Ʒ�ɶԳ��ڼ����ڿ����е�һ����̼����ȩ���к�����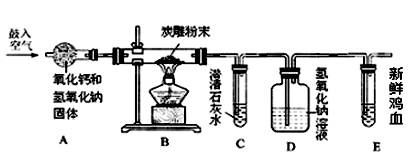
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʡ�⽭��������ѧ������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ��ƶ���
̿����ͼ����һ�ּ��������������Ϊһ��IJ�Ʒ��
 ���ֲ�Ʒ�ɶԳ��ڼ����ڿ����е�һ����̼����ȩ���к�����
���ֲ�Ʒ�ɶԳ��ڼ����ڿ����е�һ����̼����ȩ���к�����
������Ч������ij����С�����̿���Ʒ���г���̽����
[�������]̿���Ʒ���Ƿ���̼Ԫ�ء�
[���������]̿���Ʒ�к���̼Ԫ��
[��������]��̼�ڿ�������ȫȼ��ֻ���ɶ�����̼������ȫȼ��
���ɶ�����̼��һ����̼��
�����ʵ��˻���ѪҺ����һ����̼���ʺ��Ϊ���졣
[���ʵ��]ͨ������̿���Ʒȼ�ղ��֤��̿���Ʒ�к���̼Ԫ�ء���װ������������ͼ��
Aװ�õ�����Ϊ���տ����е�ˮ�Ͷ�����̼��������������ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ�鿪ʼ��Cװ�����а�ɫ������Cװ�÷�����ѧ��Ӧ����ʽΪ ��Eװ�������ʵļ�Ѫ��Ϊ����ɫ����֤�� ��
[����������]��3��С����Ϊ������A��Bװ�ü䣬���ӳ���ʯ��ˮ��Ŀ���� ��
��4��С����Ϊ��������ͼ��ʾ��װ�ã�
�滻ԭװ���е�Eװ�ã�����Ϊ��
������Ҫԭ�� ��
[��������]̿���Ʒ�к���̼Ԫ�ء�
[����̽��]�����̿���Ʒ����һ��̽�������ݣ���������Ľ��� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com