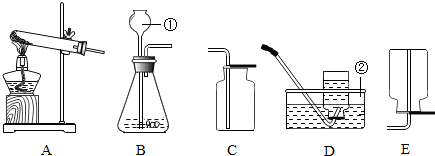
��2012?��ɽ��ͨ��һ��Ļ�ѧѧϰ�����Ѿ�������ʵ������ȡ������йع��ɣ���������ʦ�ṩ��һЩʵ��װ�ã�������ͼ�ش��������⣺

��1���ر�Aװ���е�ֹˮ�кӳ���©������ƿ��ע��һ������ˮ����ֹ����ͼ��ʾ����Aװ���Ƿ�©����
��©��
��©��
���©����������©��������ȷ��������
����A��Dװ����ȡ������̼���䷴Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��
��2������B��Dװ����ȡ�������䷴Ӧ�Ļ�ѧ����ʽΪ
��
����D�������Ƿ��ռ����ķ�����
�������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ�����������ռ���
�������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ�����������ռ���
��
��3������B��Eװ����ȡ��������ʵ�����ʱ����ͬѧ��Ϩ���˾ƾ��ƣ����ǽ����ܴ�ˮ��ȡ������ˮδ���뵼��֮ǰ���ɲ�ȡ�IJ��ȴ�ʩ�У���
Ѹ�ٽ������Ƴ�ˮ��
Ѹ�ٽ������Ƴ�ˮ��
��
Ѹ�ٽ����е��ܵ���Ƥ��ȡ��
Ѹ�ٽ����е��ܵ���Ƥ��ȡ��
��
��4��������NH
3����һ���ܶȱȿ���С�Ҽ�������ˮ�����壬��ˮ��Һ��Ϊ��ˮ������ѡ�ü����Ȼ�狀��������ƵĹ�����������ȡ����ʱ������Ϊ�ռ�������װ�����Ӧѡ��
F
F
������ĸ��ţ���ԭ���ǣ�
��ˮ�����ݳ��İ�������ֹ������ɢ�������У��Լ�����Ⱦ
��ˮ�����ݳ��İ�������ֹ������ɢ�������У��Լ�����Ⱦ
��

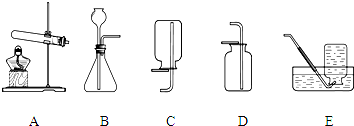
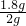 ��100%=90%����ɵõ��������̵IJ��ʱ�ʵ��ֵƫ�ͣ����ܵ�ԭ���ǣ�����ʱ��ֽ�����𣬶������̲�������ֽ�ϣ�û�л��գ�ϴ�ӡ����ʱ������������������ʧ�ˣ��ʴ�Ϊ��2H2O2
��100%=90%����ɵõ��������̵IJ��ʱ�ʵ��ֵƫ�ͣ����ܵ�ԭ���ǣ�����ʱ��ֽ�����𣬶������̲�������ֽ�ϣ�û�л��գ�ϴ�ӡ����ʱ������������������ʧ�ˣ��ʴ�Ϊ��2H2O2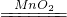 2H2O+O2���� B�� 90%�� acd
2H2O+O2���� B�� 90%�� acd 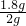 ��100%=90%����ɵõ��������̵IJ��ʱ�ʵ��ֵƫ�ͣ����ܵ�ԭ���ǣ�����ʱ��ֽ�����𣬶������̲�������ֽ�ϣ�û�л��գ�ϴ�ӡ����ʱ������������������ʧ�ˣ�����ø�����ػ����������������Ҫ���ȣ��������ܶȱȿ������ܶȴ�������ˮ��������������ſ���������ˮ���ռ���ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ���������̼�����������ǣ���һ��ȼ�ŵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ��Ϩ��֤�����ˣ���Һ��ϡ������ץס���ʵ��������䣬�����йصļ��㣮
��100%=90%����ɵõ��������̵IJ��ʱ�ʵ��ֵƫ�ͣ����ܵ�ԭ���ǣ�����ʱ��ֽ�����𣬶������̲�������ֽ�ϣ�û�л��գ�ϴ�ӡ����ʱ������������������ʧ�ˣ�����ø�����ػ����������������Ҫ���ȣ��������ܶȱȿ������ܶȴ�������ˮ��������������ſ���������ˮ���ռ���ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ���������̼�����������ǣ���һ��ȼ�ŵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ��Ϩ��֤�����ˣ���Һ��ϡ������ץס���ʵ��������䣬�����йصļ��㣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�


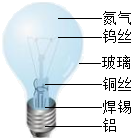 ��ͼ��������йص�����ɵ����ʣ��������ͼ�ش��������⣺
��ͼ��������йص�����ɵ����ʣ��������ͼ�ش��������⣺