
���� ��1��������������ͭ��Ӧ��������������ͭ���з�����
��2������һ����̼������ͭ�ڼ��ȵ�����������ͭ�Ͷ�����̼���з�����
��3�����ݹ��������ڶ������̵Ĵ�����������ˮ���������з�����
��4������̼������ͭ�ڸ��µ�����������ͭ�Ͷ�����̼���з�����
��5�����������������ڵ�ȼ������������ˮ���з�����
��6�����ݸ�������ڼ��ȵ���������������ء��������̺��������з�����
��� �⣺��1����������ͭ��Ӧ��������������ͭ����ѧ����ʽΪ��Fe+CuSO4=FeSO4+Cu��
��2��һ����̼������ͭ�ڼ��ȵ�����������ͭ�Ͷ�����̼����ѧ����ʽΪ��CO+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+CO2��
��3�����������ڶ������̵Ĵ�����������ˮ������������ѧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��4��̼������ͭ�ڸ��µ�����������ͭ�Ͷ�����̼����ѧ����ʽΪ��C+2CuO$\frac{\underline{\;����\;}}{\;}$2Cu+CO2����
��5�������������ڵ�ȼ������������ˮ����ѧ����ʽΪ��2H2+O2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O��
��6����������ڼ��ȵ���������������ء��������̺���������ѧ����ʽΪ��2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2����
�ʴ�Ϊ����1��Fe+CuSO4=FeSO4+Cu��
��2��CO+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+CO2��
��3��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��4��C+2CuO$\frac{\underline{\;����\;}}{\;}$2Cu+CO2����
��5��2H2+O2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O��
��6��2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2����
���� �ڽ������ʱ�����ȷ���Ӧ�õ�ԭ����Ȼ���ҳ���Ӧ����������Ϸ���ʽ����д������д����ʽ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | ���� | ��ѡ�Լ��� |
| A | CO2 | CO | ��ȼ |
| B | NaCl | Na2CO3 | �μ�������ϡ���ᡢ���� |
| C | CuO | Cu | ����������� |
| D | CaO | CaCO3 | ��ˮ�ܽ⡢���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaCl��Һ������ˮ | B�� | ϡ����ͳ���ʯ��ˮ | ||
| C�� | Na2CO3��Һ��NH4Cl��Һ | D�� | ϡ�����KCl��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������̼��ʹ�������ɫʯ����ֽ��� | |
| B�� | �������������ʹ����طֽ���������� | |
| C�� | ��������ʢװ����ͭ��Һʱ�ᱻ��ʴ | |
| D�� | ˮ�տ����װ�ˮ���dz���-�¶����ߣ����ӱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2SO4 | B�� | CuSO4 | C�� | ZnSO4 | D�� | AgNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
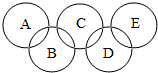 A��B��C��D��E������ľ̿��������������̼��һ����̼������ͭ�е�һ�֣�һ�������£����������ʼ��ܷ�����Ӧ����ͼ��������A����ʹ����ʯ��ˮ����ǵ����壬C������ȼ�ԣ���
A��B��C��D��E������ľ̿��������������̼��һ����̼������ͭ�е�һ�֣�һ�������£����������ʼ��ܷ�����Ӧ����ͼ��������A����ʹ����ʯ��ˮ����ǵ����壬C������ȼ�ԣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʯ | B�� | ���ʵĿ��� | C�� | �ɾ��Ķ��� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
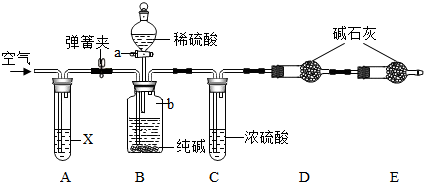
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com