��������ṩ���Լ�����������Ҫ����գ�
I���Լ���ϡ���ᡢϡ���ᡢ����������Һ������ʯ����������
��������������̨��ʯ��������ʡ�ԣ�

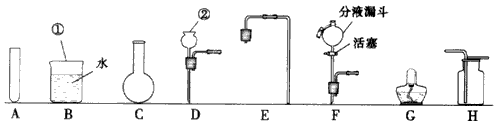
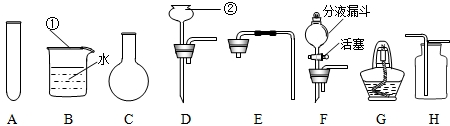
��1��д��ͼ�б��Т١������������ƣ���
�ձ�
�ձ�
����
����©��
����©��
��
��2��ʵ������ȡ����ʱ������װ�ÿ�ѡ��ͼ�е�
C��D
C��D
������ĸ������Ӧ�Ļ�ѧ����ʽΪ
���������ɸ�����ķ���Ϊ
�������ǵ�ľ���쵽����ƿ�У������ȼ����֤��������Ϊ����
�������ǵ�ľ���쵽����ƿ�У������ȼ����֤��������Ϊ����
��
��3���ø�װ�ú��ṩ���Լ���ʵ���һ�������ȡ
CO2
CO2
���壬��Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
���ռ�������ķ�����
�����ſ�����
�����ſ�����
�����������ƿ�ķ�����
��ȼ�ŵ�ľ������ƿ�ڣ�����Ϩ��֤����ƿ
��ȼ�ŵ�ľ������ƿ�ڣ�����Ϩ��֤����ƿ
���������ķ����ǣ���������ͨ��
�����ʯ��ˮ
�����ʯ��ˮ
��������
�����ʯ��ˮ�����
�����ʯ��ˮ�����
��Ӧ�Ļ�ѧ����ʽΪ
CO2+Ca��OH��2=CaCO3��+H2O
CO2+Ca��OH��2=CaCO3��+H2O
��
��4�����һ����ȡ��������ˮ�ļ���װ�ã���ѡ��ͼ�е�A��B��
C��E��G
C��E��G
������ĸ����