

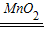 2H2O+O2����
2H2O+O2����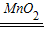 2H2O+O2����Һ����������ɫ�������ܽ⣬����ɫ���ݲ�����
2H2O+O2����Һ����������ɫ�������ܽ⣬����ɫ���ݲ�����

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
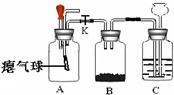
 ��ʦ����ͼ��ʾװ��Ϊͬѧ������������Ȥʵ�飮
��ʦ����ͼ��ʾװ��Ϊͬѧ������������Ȥʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
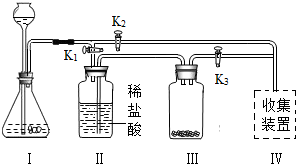
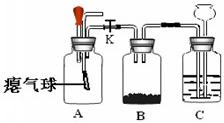 32����ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�飮Aװ����װ�������ԼΪ3��1�Ŀ����Ͷ�����̼�Ļ������Bװ����ʢ���������ۣ�Cװ����ʢ��������ϡ���ᣮ
32����ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�飮Aװ����װ�������ԼΪ3��1�Ŀ����Ͷ�����̼�Ļ������Bװ����ʢ���������ۣ�Cװ����ʢ��������ϡ���ᣮ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
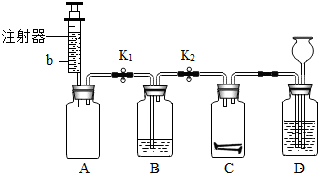 ��2012?������һģ����ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�飮Aװ�ü���ƿ��װ�������ԼΪ1��1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᣮ
��2012?������һģ����ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�飮Aװ�ü���ƿ��װ�������ԼΪ1��1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᣮ�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com