
ČēĶ¼ŹĒĢ½¾æ·Ö×ÓŌĖ¶ÆµÄŹµŃ锣ĻĀĮŠĻÖĻóÓė½įĀŪ“ķĪóµÄŹĒ(””””)

A. ÅØŃĪĖį¾ßÓŠ»Ó·¢ŠŌ
B. ·Ö×ÓŌŚ²»¶ĻŌĖ¶Æ
C. ŅŅÉÕ±ČÜŅŗµÄŃÕÉ«»įøıä
D. ŅŅ”¢±ūÉÕ±ÄŚČÜŅŗµÄŃÕÉ«»įøıä
D ”¾½āĪö”æA”¢Ņ»¶ĪŹ±¼äŗóŅŅÉÕ±ÖŠµÄ·ÓĢŖČÜŅŗŃÕÉ«±äĪŖĪŽÉ«£¬Į½Õßƻӊֱ½Ó½Ó“„£¬ĖµĆ÷ÅØŃĪĖįÓŠ»Ó·¢ŠŌ£¬ÕżČ·£»B”¢ĀČ»ÆĒā·Ö×ÓŌŚ²»¶ĻŌĖ¶Æ£¬²»¶ĻĻņĒāŃõ»ÆÄĘČÜŅŗÖŠĄ©É¢£¬ŅŅÉÕ±ÖŠČÜŅŗŃÕÉ«±äĪŖĪŽÉ«£¬ĖµĆ÷ĮĖ·Ö×ÓŌŚ²»¶ĻŌĖ¶Æ£¬ÕżČ·£»C”¢ĒāŃõ»ÆÄĘČÜŅŗŹ¹·ÓĢŖŹŌŅŗ±äĪŖŗģÉ«£¬ÅØŃĪĖį¾ßÓŠ»Ó·¢ŠŌ£¬ĀČ»ÆĒā·Ö×ÓŌŚ²»¶ĻŌĖ¶Æ£¬²»¶ĻĻņĒāŃõ»ÆÄĘČÜŅŗÖŠĄ©É¢£¬Ėį¼īÖŠŗĶ£¬ŅŅÉÕ±ÖŠČÜŅŗŃÕÉ«±äĪŖĪŽÉ«£¬ÕżČ·£»D”¢ĀČ»ÆĒā·Ö×ÓŌŚ²»¶ĻŌĖ¶Æ£¬²»¶ĻĻņĒāŃõ»ÆÄĘČÜ... Ńō¹āæ¼³”µ„ŌŖ²āŹŌ¾ķĻµĮŠ“š°ø
Ńō¹āæ¼³”µ„ŌŖ²āŹŌ¾ķĻµĮŠ“š°ø ĆūŠ£ĮŖĆĖ³å“Ģ¾ķĻµĮŠ“š°ø
ĆūŠ£ĮŖĆĖ³å“Ģ¾ķĻµĮŠ“š°ø ĆūŠ£Ģį·ÖŅ»¾ķĶØĻµĮŠ“š°ø
ĆūŠ£Ģį·ÖŅ»¾ķĶØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗĖÄ“ØŹ”°ĶÖŠŹŠ2018ÄźÖŠæ¼Ąķ×Ū£Ø»Æѧ²æ·Ö£©ŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĶ¼Ź¾ĪŖ”°ÖŠ¹ś½ŚÄÜ”±±źÖ¾µÄŹĒ£Ø £©

A. A B. B C. C D. D
D ”¾½āĪö”æA”¢»ŲŹÕ±źÖ¾£»B”¢½ŚĖ®±źÖ¾£»C”¢½ūÖ¹ŃĢ»š£»D”¢½ŚÄܱźÖ¾”£¹ŹŃ”D”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗŗÓÄĻŹ”2018½ģ¾ÅÄź¼¶ÕŠÉśÄ£Äāæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
ÉĮÖ»šŌÖŹ±³£æ³·„Ņ»ĢõøōĄė“ų£¬ÕāŃł×öŅĄ¾ŻµÄĆš»šŌĄķŹĒ_________”£Éś»īÖŠ³£ÓƵÄÓ²Ė®Čķ»ÆµÄ·½·ØŹĒ_________”£
Ēå³żæÉČ¼Īļ Öó·Š ”¾½āĪö”æøł¾ŻĆš»šµÄŌĄķ¼°Ó²Ė®Čķ»ÆµÄ·½·Ø·ÖĪö½ā“š”£ÉĮÖ»šŌÖŹ±³£æ³·„Ņ»ĢõøōĄė“ų£¬ÕāŃł×öŅĄ¾ŻµÄĆš»šŌĄķŹĒĒå³żæÉČ¼Īļ£»Éś»īÖŠ³£ÓƵÄÓ²Ė®Čķ»ÆµÄ·½·ØŹĒÖó·Š”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗŠĀ½®2018ÄźÖŠæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗ¼ĘĖćĢā
ij»ÆѧŠĖȤŠ”×éČ”12.5æĖµÄ“óĄķŹÆѳʷ(ŌÓÖŹ²»ÓėŃĪĖį·“Ó¦)¼ÓČėµ½Ņ»¶ØĮæµÄĻ”ŃĪĖįÖŠ£¬²śÉśCO2µÄÖŹĮæÓėĻ”ŃĪĖįµÄÖŹÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾”£Ēė¼ĘĖć£ŗ

(1)Éś³ÉCO2µÄÖŹĮæŹĒ_____g”£
(2)øĆŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹżĪŖ__________(¼ĘĖć½į¹ū±£Įæ0.1%)”£
4.4 7.3% ”¾½āĪö”æ±¾Ģāæ¼²éĮĖ»Æѧ·“Ó¦·½³ĢŹ½ÓėČÜÖŹÖŹĮæ·ÖŹżĻą½įŗĻµÄ¼ņµ„¼ĘĖć”£½āĢāµÄ¹Ų¼üŹĒ¶Į¶®¹ŲĻµĶ¼ÖŠµÄŹż¾ŻÓė»Æѧ·“Ó¦µÄ¹ŲĻµ”£ (1)øł¾Ż¹ŲĻµĶ¼æÉÖŖ£¬Éś³ÉµÄ¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ4.4g£» (2)Éč£ŗøĆŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹżĪŖx CaCO3+2HClØTCaCl2+H2O+CO2”ü 73 44 100g”Įx 4.4g x=7.3% “š£ŗ£Ø1£©Éś³ÉCO2µÄÖŹĮæ...²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗŠĀ½®2018ÄźÖŠæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĶ¼ĻóÄÜÕżČ··“Ó³Ęä¶ŌÓ¦±ä»Æ¹ŲĻµµÄŹĒ(””””)
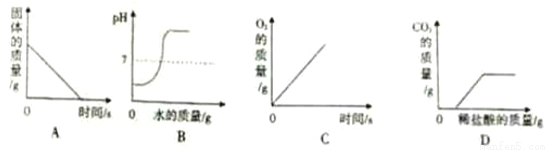
A. ¼ÓČČŅ»¶ØÖŹĮæµÄKMnO4¹ĢĢå
B. ĻņŹ¢ÓŠÉŁĮæH2SO4ČÜŅŗµÄÉÕ±ÖŠµĪ¼ÓŅ»¶ØÖŹĮæµÄĖ®
C. ¼ÓČČŅ»¶ØÖŹĮæKClO3ŗĶMnO2µÄ»ģŗĻĪļ
D. ĻņŹ¢ÓŠŅ»¶ØÖŹĮæNaOHŗĶNa2CO3»ģŗĻČÜŅŗµÄÉÕ±ÖŠµĪ¼ÓĻ”ŃĪĖį
D ”¾½āĪö”æA”¢øßĆĢĖį¼ŲŌŚ¼ÓČČĢõ¼žĻĀÉś³ÉĆĢĖį¼Ų”¢¶žŃõ»ÆĆĢŗĶŃõĘų£¬Ź£Óą¹ĢĢåµÄÖŹĮæ²»¶Ļ¼õÉŁ£¬ÖĮĶźČ«·“Ó¦£¬Ź£Óą¹ĢĢåµÄÖŹĮæ²»ŌŁ¼õÉŁ£¬×īŗóŹ£ÓąµÄ¹ĢĢåĪŖĆĢĖį¼ŲŗĶ¶žŃõ»ÆĆĢµÄ»ģŗĻĪļ£¬²»æÉÄÜĪŖ0£¬“ķĪó£»B”¢ĻņŅ»¶ØĮæµÄĮņĖįČÜŅŗÖŠ²»¶Ļ¼ÓĖ®Ļ”ŹĶ£¬ČÜŅŗŹ¼ÖÕĻŌĖįŠŌ£¬ČÜŅŗŹ¼ÖÕĻŌĖįŠŌ£¬pH²»æÉÄÜ“óÓŚ7£¬“ķĪó£»C”¢ĀČĖį¼ŲŌŚ¶žŃõ»ÆĆĢµÄ“ß»Æ×÷ÓĆĻĀŌŚ¼ÓČČĢõ¼žĻĀÉś³ÉĀČ»Æ¼ŲŗĶŃõĘų£¬ÖĮĶźČ«·“Ó¦£¬ŃõĘųµÄÖŹĮæ²»ŌŁ·¢Éśøı䣬ĘųĢåµÄÖŹĮæ“ÓĮćæŖŹ¼...²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗø£½ØŹ”2018ÄźÖŠæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗ¼ĘĖćĢā
Ņ»ÖÖŠĀŠĶ”°ČĖŌģŹ÷Ņ¶”±æÉĪüŹÕ¶žŃõ»ÆĢ¼²¢×Ŗ»ÆĪŖŅŅ“¼(C2H5OH)Č¼ĮĻ£¬»Æѧ·½³ĢŹ½ĪŖ2CO2+3H2O C2H5OH +3O2”£ŃŠ¾æĻŌŹ¾£¬Ņ»Éż”°ČĖŌģŹ÷Ņ¶”±ĆæĢģæÉ“ÓæÕĘųÖŠĪüŹÕ968gCO2”£
C2H5OH +3O2”£ŃŠ¾æĻŌŹ¾£¬Ņ»Éż”°ČĖŌģŹ÷Ņ¶”±ĆæĢģæÉ“ÓæÕĘųÖŠĪüŹÕ968gCO2”£
(1)Ņ»Éż”°ČĖŌģŹ÷Ņ¶”±¹¤×÷Ņ»ĢģæɵƵ½ŅŅ“¼µÄÖŹĮæŹĒ________?
(2)ČōĆæĢģŅ»æĆŹ÷Ę½¾łæÉĪüŹÕ48.4gCO2£¬ŌņŅ»Éż”°ČĖŌģŹ÷Ņ¶”±ĪüŹÕµÄCO2Ļąµ±ÓŚ_______æĆŹ÷ĪüŹÕµÄCO2”£
506g 20 ”¾½āĪö”æøł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½¼ĘĖć½ā“š”£(1)ÉčŅ»Éż”°ČĖŌģŹ÷Ņ¶”±¹¤×÷Ņ»ĢģæɵƵ½ŅŅ“¼µÄÖŹĮæĪŖx”£ 2CO2+3H2OC2H5OH+3O2 88 46 968g x x=506g (2)968g”Ā48.4g=20 “š£ŗ(1)Ņ»Éż”°ČĖŌģŹ÷Ņ¶”±¹¤×÷Ņ»ĢģæɵƵ½ŅŅ“¼µÄÖŹĮæŹĒ506g£»(2)ČōĆæĢģŅ»æĆŹ÷Ę½¾łæÉĪüŹÕ48.4gCO2£¬ŌņŅ»Éż”°ČĖŌģŹ÷Ņ¶”±ĪüŹÕµÄ...²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗø£½ØŹ”2018ÄźÖŠæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠŹµŃé·½°øÄÜ“ļµ½ŹµŃéÄæµÄµÄŹĒ

A. A B. B C. C D. D
D ”¾½āĪö”æA”¢¼ģŃéij¹ĢĢåĪŖĢ¼ĖįŃĪ£¬Č”Ńł£¬µĪ¼ÓŃĪĖį£¬¹Ū²ģŹĒ·ńÓŠĘųÅŻ£¬²¢½«ĘųĢåĶØČė³ĪĒåŹÆ»ŅĖ®ÖŠ£¬æ“ŹÆ»ŅĖ®ŹĒ·ń±ä»ė×Ē£¬½öĶعżµĪ¼ÓŃĪĖį£¬¹Ū²ģŹĒ·ńÓŠĘųÅŻ²»Äܼų±šŹĒĢ¼ĖįŃĪ£¬Ņ²æÉÄÜŹĒ»īĘĆ½šŹōÓėŃĪĖį·“Ó¦£¬“ķĪó£»B”¢Ö¤Ć÷¶žŃõ»ÆĢ¼ÄÜÓėĖ®·“Ó¦£¬Ó¦½«¶žŃõ»ÆĢ¼ĶØČėµĪÓŠ×ĻÉ«ŹÆČļČÜŅŗµÄĖ®ÖŠ£¬ŅņĪŖ·ÓĢŖÓöĖįŗĶÖŠŠŌČÜŅŗ²»±äÉ«£¬“ķĪó£»C”¢¼ģŃéŃõĘųŅŃŹÕ¼ÆĀś£¬Ó¦½«“ų»šŠĒµÄľĢõ·ÅŌŚ¼ÆĘųĘææŚ£¬“ķĪó£»D”¢Ö¤Ć÷ĄÆÖņÖŠŗ¬ÓŠĢ¼ŌŖĖŲ£¬½«ÄŚ±ŚÕŗÓŠ...²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗŗÓ±±Ź”2018ÄźÖŠæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠŹĀŹµ²»ÄÜ×÷ĪŖĻąÓ¦¹ŪµćµÄÖ¤¾ŻµÄŹĒ( )
A. ³¾ĶĮ·ÉŃļ£¬ĖµĆ÷·Ö×ÓŹĒŌĖ¶ÆµÄ
B. µē½āĖ®µĆµ½ĒāĘųŗĶŃõĘų£¬ĖµĆ÷·Ö×ÓŹĒæÉ·ÖµÄ
C. ĘųĢå±»Ń¹ĖõŗóĢå»ż·¢ÉśĮĖ½Ļ“ó±ä»Æ£¬ĖµĆ÷ĘųĢå·Ö×Ó¼ä¾ą½Ļ“ó
D. ½«Į½øöøɾ»Ę½ÕūµÄĒ¦Öł½ōŃ¹ŌŚŅ»Ęš»į½įŗĻĘšĄ“£¬ĖµĆ÷·Ö×Ó¼ä“ęŌŚŅżĮ¦
A ”¾½āĪö”æ A”¢³¾ĶĮŹĒŗź¹ŪĪļÖŹ£¬²»ŹĒĪ¢¹ŪĪļÖŹ£¬²»ÄÜÓĆĪ¢¹ŪĮ£×ÓĄ“½āŹĶĻÖĻ󣬷ūŗĻĢāŅā£»B”¢µē½āĖ®µĆµ½ĒāĘųŗĶŃõĘų£¬ĖµĆ÷»Æѧ±ä»ÆÖŠ·Ö×ÓŹĒæɷֵģ¬Ō×Ó²»æÉ·Ö£¬²»·ūŗĻĢāŅā£»C”¢ĘųĢå±»Ń¹ĖõŗóĢå»ż·¢ÉśĮĖ½Ļ“ó±ä»Æ£¬ĖµĆ÷ĘųĢå·Ö×Ó¼ä¾ą½Ļ“󣬲»·ūŗĻĢāŅā£»D”¢½«Į½øöøɾ»Ę½ÕūµÄĒ¦Öł½ōŃ¹ŌŚŅ»Ęš»į½įŗĻĘšĄ“£¬ĖµĆ÷·Ö×Ó¼ä“ęŌŚŅżĮ¦£¬²»·ūŗĻĢāŅā”£¹ŹŃ”A”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗɽ¶«Ź”ĒąµŗŹŠ2018ÄźÖŠæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗ¼ņ“šĢā
ŹµŃéŹĒ½ųŠŠæĘѧĢ½¾æµÄÖŲŅŖŹÖ¶Ī”£Ēė»Ų“šĻĀĮŠĪŹĢā”£

£Ø1£©ŹµŃé A ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_______£¬µ±µēŌ“½ÓĶØŅ»¶ĪŹ±¼äŗó£¬a ¹ÜÓė b ¹ÜÖŠĘųĢåµÄĢå»ż±ČŌ¼ĪŖ______”£
£Ø2£©ŹµŃé B ¼ÆĘųĘæČװĀśĖ®µÄÄæµÄŹĒ______.
£Ø3£©ŹµŃé C Ķعż______ĻÖĻó£¬æÉµĆ³öŃõĘųŌ¼Õ¼æÕĘųĢå»ż 1/5 µÄ½įĀŪ”£
£Ø4£©ŹµŃé D ÖŠĖ®µÄ×÷ÓĆŹĒ______”£
2H2O2H2”ü+O2”ü£» 1:2£» ·ĄÖ¹ŹÕ¼ÆµÄŃõĘų²»“棻 ĮæĶ²ÖŠµÄĖ®½ųČė¼ÆĘųĘæÖŠ£¬Ō¼Õ¼¼ÆĘųĘæÖŠŌæÕĘųĢå»żµÄĪå·ÖÖ®Ņ»£» ·ĄÖ¹øßĪĀČŪČŚĪļ½¦Ā䣬ÕØĮŃ¼ÆĘųĘæµ× ”¾½āĪö”æ£Ø1£©øł¾ŻĖ®ŌŚĶصēµÄĢõ¼žĻĀ·Ö½āÉś³ÉĒāĘųŗĶŃõĘų½ā“š£»øł¾Żµē½āĖ®ŹµŃéµÄ½įĀŪ½ā“š£»£Ø2£©øł¾Ż·ĄÖ¹ŹÕ¼ÆµÄŃõĘų²»“æ½ā“š£»£Ø3£©øł¾ŻĮæĶ²ÖŠµÄĖ®½ųČė¼ÆĘųĘæÖŠ£¬Ō¼Õ¼¼ÆĘųĘæÖŠŌæÕĘųĢå»żµÄĪå·ÖÖ®Ņ»½ā“š£»£Ø4£©øł¾Ż·ĄÖ¹øßĪĀČŪČŚĪļ½¦Ā䣬ÕØĮŃ¼ÆĘųĘæµ×½ā“š”££Ø...²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com