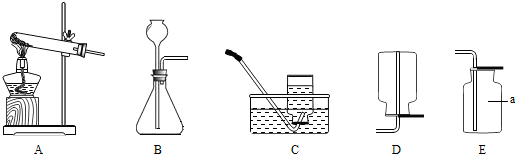
��1����������װ��ͼ���ش��й����⣺
��ʵ������ȡCO
2ʱ��ѡ�õķ������ռ�װ���ǣ���װ����ĸ��
B��C
B��C
����ȡCO
2��װ�û���������ȡ
O2
O2
����һ�����廯ѧʽ������Ӧ�Ļ�ѧ����ʽΪ
��
����ͼF��ʾ�����ձ����㵹������̼���ɹ۲쵽��������
�²�������Ϩ���ϲ������Ϩ��
�²�������Ϩ���ϲ������Ϩ��
��˵��������̼���е�������
CO2����ȼ�գ�Ҳ��֧��ȼ�գ��ܶȱȿ�����
CO2����ȼ�գ�Ҳ��֧��ȼ�գ��ܶȱȿ�����
��
�ۻ�ѧ��ȤС��������ϵ�֪�����ȹ���̼�����ƻ����̼����臨��ܲ���CO
2��
�仯ѧ����ʽ�ֱ��ǣ�
2NaHCO
3Na
2CO
3+H
2O+CO
2��
NH
4HCO
3NH
3��+H
2O+CO
2��
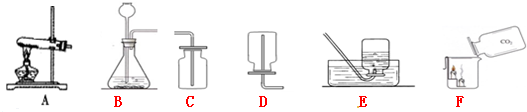
ijͬѧ����������һ����ѧ��Ӧ��ȡCO
2��Ӧ��ѡ�õķ���װ����
A
A
�������������ڶ�����ѧ��Ӧ��ȡCO
2��������
�����������������壬�Ƶõ�CO2����
�����������������壬�Ƶõ�CO2����
��
��2��С��ͬѧ����100g������������Ϊ20%��NaCl��Һ��������������ͼ��
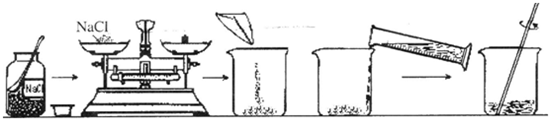
��С��ͬѧӦ��ȡ�Ȼ��Ƶ�������
20g
20g
��
��ָ��ͼ�е�һ������
ƿ��������
ƿ��������
��
������������ƽ��ȡʳ�εĹ����У�����ָ��ƫ��ֶ����Ҳ࣬���������IJ�����
C
C
������ţ���
A���������ϼ����Ȼ��ƣ�ֱ����ƽƽ�� B���������ϼ������룬ֱ����ƽƽ��
C���������ϼ��������Ȼ��ƣ�ֱ����ƽƽ�� D���ƶ����룬ֱ����ƽƽ��
�����С�����Ƶ��Ȼ�����Һ��������������С��20%������ɴ����Ŀ���ԭ����
����ǰ��ƽû�е�ƽ�������ֽ�ϲ�������ʳ�Σ��������ɣ�
����ǰ��ƽû�е�ƽ�������ֽ�ϲ�������ʳ�Σ��������ɣ�
����һ�֣���