
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
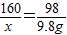

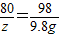
 =160g
=160g

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
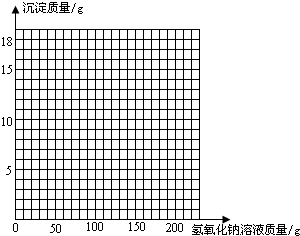
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ���� | �¶� | ����ʯ | ����Ũ�� | ʵ��̽��Ŀ�� |
| �� | 20�� | �ֿ��� | 5% | ��ʵ��ٺ͢�̽��Ũ�ȶԷ�Ӧ������Ӱ�죮 ��ʵ��ں� ��ʵ��ٺ͢�̽������ʯ��ϸ�Է�Ӧ������Ӱ�죮 |
| �� | 20�� | �ֿ��� | ||
| �� | ϸ���� | 5% | ||
| �� | 40�� | 10% |
| ���� | ���Ƿ���� | ˵�����ɣ�д��ѧ����ʽ�� |
| ����Һ��pH�Ƿ�С��7 | ���� | �Ȼ�����������Һ������������ԣ� |
| �μ���������Һ�۲��Ƿ��а�ɫ�������� | ||
| �����۹۲��Ƿ������� |
| ����̼������Һ������/g | 40.0 | 80.0 | 120.0 | 160.0 | 200.0 |
| ���ɳ���������/g | 0.0 | 3.0 | 7.0 | 10.0 | 10.0 |
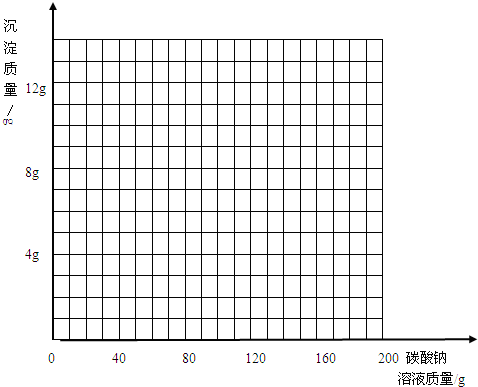
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����NaOH��Һ������/g | 20.0 | 40.0 | 60.0 | 80.0 | 100.0 |
| ���ɳ���������/g | 0.0 | 0.00 | 2.45 | 4.90 | 4.90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com