28�������д����л�ѧ����ѧ������������ʶ���������磬�ٽ���ᷢչ��
��1��������һ��Ӫ����ֵ�ߡ����ܴ�һ�ӭ��ʳƷ���±��Ƕ�������Ҫ�ɷֵ�ƽ������������

�����к��е�Ӫ���س������ʡ���֬���������
ˮ�����Ρ�ά����
��
��2��ij��ֽ������Ӫ��ר�����������ر��Ƽ���һ�����ʳ��Ϊ��һ����ţ�̣�һ����������������ͷ���������������߲ˡ�ˮ���ȣ�

����˵����ȷ����
BC
������ţ���
A���߲ˡ�ˮ����ֻ��һ��Ӫ����
B����������ʳƷ��Ҫ���̡������㡢���
C������������������Ҫ��Դ����ͷ��������ʳƷ�к��е���������
��3�����������е�������������ȷ����
A
������ţ���
A���ü�ȩˮ��Һ����ˮ��Ʒ�Է�ֹ����
B���ӷ���ˮ������۲���ĭ�Ķ�������Ӳˮ����ˮ
C�������ա�����ζ�ķ���������ë��ά�͵���֯�ɵ�����
��4���±��Ǽ��ֿ�ȼ����ѹǿΪ101kPaʱ�ķе㣺
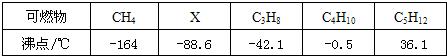
�������ҳ��ϱ�������������ϵĹ���
����̼����Ԫ����ɻ������̼����ԭ�Ӹ�����Ϊn����2n+2��
�����ݷ���˼����ȷ��X�Ļ�ѧʽΪ
C2H6
��
�ڴ�����ȼ���Լ�ѹ������Һ������ѹ���أ�Һ��������������������ȼ�գ��ϱ��п�������ȼ�ϵ���
C4H10
��д��ѧʽ����







 ȼ������Ҫ����Դ��
ȼ������Ҫ����Դ��