���𰸡�
��������1���ٸ���Ԫ�ػ��ϼ۵ı�ʾ�������ڸ�Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں��н��
�ڸ��ݱ������ӷ���ǰ������ֱ�ʾ���Ӹ�����
����������˫ԭ�ӹ��ɣ����仯ѧʽǰ������Ӧ�����ֱ�ʾ���Ӹ��������н��
�ܸ���ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣����н��
��2���������⣬�ٸ������������ļ� Ϊ�������ƣ�
����ʹʪ���ɫʯ����ֽ����������Ϊ������
���������������������������������
�ܳ�����ʳƷ�����������Ϊ�����ƣ����ݻ����ﻯѧʽ����д���裺������������ۡ������Լ���н��
��3��������д��ѧ����ʽ�IJ��裺д���䡢ע���ȣ���ȷ��д��ѧ����ʽ���ɣ�
����⣺��1���ٸ���Ԫ�ػ��ϼ۵ı�ʾ�������ڸ�Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں���ˣ�+2�۵�ͭԪ�ر�ʾΪ��
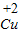
��
�ڸ��ݱ������ӷ���ǰ������ֱ�ʾ���Ӹ��������2NO
3-��ʾ��2����������ӣ�
����������˫ԭ�ӹ��ɣ���ѧʽΪ��O
2�����仯ѧʽǰ������Ӧ�����ֱ�ʾ���Ӹ��������2�������ӱ�ʾ
��2O
2��
�ܸ���ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣����3����ԭ�ӱ�ʾΪ��3Ag��
��2���������⣬�ٸ������������ļ� Ϊ�������ƣ���+2�۵ĸ�Ԫ�غ�-1�۵����������ɣ����ݻ����ﻯѧʽ����д���裬�������ƵĻ�ѧʽΪ��Ca��OH��
2��
����ʹʪ���ɫʯ����ֽ����������Ϊ������ ��+1�۵���Ԫ�غ�-3�۵ĵ�Ԫ����ɣ����ݻ����ﻯѧʽ����д���裬�����Ļ�ѧʽΪ��NH
3��
�����������������������Ϊ���������� ������������������д��������Ԫ�����ң���һ��Ԫ���������½DZ����Ԫ��ԭ�Ӹ�����������������ѧʽΪ��NO
2��
�ܳ�����ʳƷ�����������Ϊ�����ƣ���+2�۵ĸ�Ԫ�غ�-2�۵���Ԫ�ع��ɣ����ݻ����ﻯѧʽ����д���裬�����ƵĻ�ѧʽΪ��CaO��
��3��������д��ѧ����ʽ�IJ��裻
����ɫֲ����й�����ã���������̼��ˮ��Ҷ�����й������������Ǻ�����������ʽΪ��
6CO
2+6H
2O
C
6H
12O
6+6O
2��
����Al��OH��
3����θ������ʽΪ��Al��OH��
3+3HCl=AlCl
3+3H
2O����
��������ͭʪ����ͭ �ķ���ʽΪ��Fe+CuSO
4=FeSO
4+Cu��
�ܼ���ȼ�����ɶ�����̼��ˮ�ķ���ʽΪ��CH
4+2O
2 
CO
2+2H
2O��
�ʴ�Ϊ��
��1����
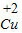
�� ��2����������ӣ� ��2O
2�� ��3Ag��
��2����Ca��OH��
2�� ��NH
3�� ��NO
2�� ��CaO��
��3����6CO
2+6H
2O
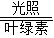
C
6H
12O
6+6O
2��
��Al��OH��
3+3HCl=AlCl
3+3H
2O��
��Fe+CuSO
4=FeSO
4+Cu��
��CH
4+2O
2 
CO
2+2H
2O��
���������⿼��ѧ��ѧ���Ի�ѧ�����ѧ����ʽ����д������������ע�ػ�����

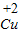 ��
�� 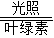 C6H12O6+6O2��
C6H12O6+6O2��  CO2+2H2O��
CO2+2H2O��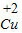 �� ��2����������ӣ� ��2O2�� ��3Ag��
�� ��2����������ӣ� ��2O2�� ��3Ag��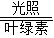 C6H12O6+6O2��
C6H12O6+6O2�� CO2+2H2O��
CO2+2H2O��