������ŷɴ�����ɹ�ʹ�ҹ���Ϊ�����ϵ��������������Ա����̫�յĹ��ң�ÿһ���й��˶�Ϊ�˶��е�������
��1��һ�������ͷɴ��Ļ���ƽ�����ʢ��Һ̬�£�N2H4����Һ̬˫��ˮ��H2O2�������ǻ��ʱ�ڸ��¸�ѹ�����ɵ�����ˮ�����µ���Է��������� ����Ԫ�ص�������Ϊ ��Һ̬�º�Һ̬˫��ˮ������ѧ��Ӧ�ķ���ʽ�� ��
��2����������š����˷ɴ���ʹ�õĺ�����������ͨ���������кܴ������һ��ij���������̼�⻯�����������������̼������������ߵ������ܶ�����ͨ����Ҫ�õö࣬�ܶ�Ҳ����ͨ��Ҫ������ܹ����õ���Ӧ���Ӷ���̫�ջ���������˵����ȷ���ǣ� ���������գ���
���������͵ijɷ���ͬ�����������͵��������ʲ���ϴ�
�����˷ɴ���ʹ�ú�����������Ϊ���Ļ�ѧ�����ȶ���
���𰸡���������1���ٸ����µĻ�ѧʽ��N2H4����֪���µ���Է�������=���������ԭ������×��ԭ�Ӹ�����+��������ԭ������×��ԭ�Ӹ�������
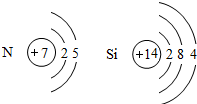
�����и�Ԫ�ص�������Ϊ N��H=���������ԭ������×��ԭ�Ӹ���������������ԭ������×��ԭ�Ӹ�������
��Һ̬�º�Һ̬˫��ˮ������ѧ��Ӧ�����ɵ�����ˮ���ݴ�д������ʽ���ɣ�
��2�������������͵����ʽ��з���ѡ��
����⣺��1���ٸ����µĻ�ѧʽ��N2H4����֪���µ���Է�������=14×2+4=32��
�����и�Ԫ�ص�������Ϊ N��H=��14×2������1×4��=28��4=7��1��
��Һ̬�º�Һ̬˫��ˮ������ѧ��Ӧ�ķ���ʽΪ��N2H4+2H2O2=N2+4H2O��
��2�����������͵ijɷֲ���ͬ��һ����̼���⣬һ����̼�ͷ����ʢٲ��ʺϣ�
������֪������̼������������ߵ������ܶ�����ͨ����Ҫ�õö࣬�ܶ�Ҳ����ͨ��Ҫ��֪���������͵��������ʲ���ϴʢ��ʺϣ�
�۷��м�ǿ����ȼ�ԣ������������������γ��ȶ�����λ������ʢ��ʺϣ�
��ѡ���ڢۣ�
�ʴ�Ϊ����1��32��N��H=7��1��N2H4+2H2O2=N2+4H2O��
��2���ڢۣ�
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������



 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�