
| ��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� |
| ȡ��Ʒ������g�� | 50.0 | 50.0 | 50.0 | 50.0 |
| ȡϡ����������g�� | 40.0 | 80.0 | 120.0 | 160.0 |
| ��������������g�� | 0.4 | 0.8 | 1.0 | 1.0 |
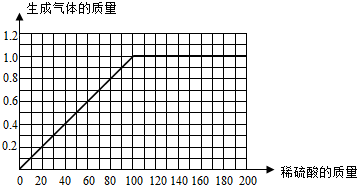
| 65 |
| x |
| 2 |
| 1.0g |
| 32.5g |
| 50g |



 �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Ϸ�Ӧ | B���ֽⷴӦ |
| C���û���Ӧ | D�����ֽⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ������ | �� | ̼ | �� | �� | �� | �� |
| Ԫ�ط��� | H | C | O | Cl | Na | Ca |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 1926�꣬�ҹ�������ѧ�Һ�°�����������һ�ָ�Ч�����Ĵ������������������Ƽ�����ֳƺ����Ƽ�������������������з�Ӧ��
1926�꣬�ҹ�������ѧ�Һ�°�����������һ�ָ�Ч�����Ĵ������������������Ƽ�����ֳƺ����Ƽ�������������������з�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | NaOH��Һ��������g�� | ϡ����������m L�� | ������ù���������g�� |
| 1 | 80.00 | 100.00 | 11.70 |
| 2 | 80.00 | 80.00 | 11.70 |
| 3 | 80.00 | 60.00 | 11.43 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����˿�ڿ�����ȼ�գ��������䣬���ɺ�ɫ���� |
| B������������ȼ�գ���������ɫ���� |
| C����ʢ��Ũ������Լ�ƿ��ƿ����ƿ�ڳ��ְ��� |
| D������������ͨ����ɫʯ����Һ�У���Һ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com