ijƷ���̷۱�ǩ��ʾ�������ʺ���Ϊ18%����Ԫ�غ���Ϊ2.88%�������̷��еĵ�Ԫ��ȫ�����Ե����ʣ���ȡ100g���̷���Ʒ����ʹ���е������еĵ�Ԫ��ȫ��ת��ɰ�����NH3��������50gϡ���Ὣ����ǡ��������[2NH3+H2SO4�T��NH4��2SO4]����ʱ��Һ������Ϊ51.7g��������������һλС������
��1����ǩ��ʾ�ĺ������Ƿ���ʵ��______�������ǣ�ֻ�м���ʽ�� ______��
��2������ϡ������Һ���������������Ϊ���٣�
���𰸡�
���������������غ㶨�ɵó����ɵİ�����������Ȼ����ݵ�Ԫ�ص������غ�Ϳ�������̷��еĺ������Ƿ���ʵ�����ݰ��������������йصĻ�ѧ����ʽ�Ϳ�����йص�δ֪����
����⣺��1������Ԫ�ص��������غ㶨�ɿ�֪�������еĵ�Ԫ�ص�����Ӧ�����̷۵ĵ������е�Ԫ�ص���������Ԫ�ص�����Ϊ��
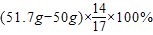
=1.4g����˸��̷��е�Ԫ�ص���������Ϊ
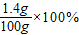
=1.4%��2.88%��
�ʴ�Ϊ������ʵ��
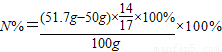
��2����50g��Һ�����������Ϊx
2NH
3+H
2SO
4�T��NH
4��
2SO
4
34 98
1.7g x
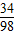
=
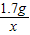
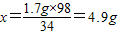
�����õ�������Һ�����ʵ���������Ϊ
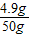
×100%=9.8%
�����������ѶȲ��Ǻܴ���Ҫ�����������غ㶨�ɵ�Ӧ�ú��ݻ�ѧ����ʽ�����йصļ��㣬����ѧ���ķ��������ͽ�������������
��Ŀ�����л�ѧ
��Դ��2009-2010ѧ��������������ʢ����ѧ���꼶���ϣ���ѧ���Ծ���12�·ݣ��������棩
���ͣ������
ijƷ���̷۱�ǩ��ʾ�������ʺ���Ϊ18%����Ԫ�غ���Ϊ2.88%�������̷��еĵ�Ԫ��ȫ�����Ե����ʣ���ȡ100g���̷���Ʒ����ʹ���е������еĵ�Ԫ��ȫ��ת��ɰ�����NH3��������50gϡ���Ὣ����ǡ��������[2NH3+H2SO4�T��NH4��2SO4]����ʱ��Һ������Ϊ51.7g��������������һλС������
��1����ǩ��ʾ�ĺ������Ƿ���ʵ��______�������ǣ�ֻ�м���ʽ�� ______��
��2������ϡ������Һ���������������Ϊ���٣�
�鿴�𰸺ͽ���>>

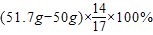 =1.4g����˸��̷��е�Ԫ�ص���������Ϊ
=1.4g����˸��̷��е�Ԫ�ص���������Ϊ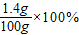 =1.4%��2.88%��
=1.4%��2.88%��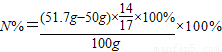
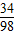 =
=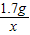
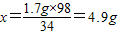
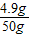 ×100%=9.8%
×100%=9.8%

