
Ζ÷Έω Θ®1Θ©ΗυΨί”ΟΖ ‘μΥ°Φλ―ι”≤Υ°Μρ»μΥ°Ϋβ¥πΘΜ
Θ®2Θ©ΗυΨίœ¥ΫύΨΪΨΏ”–»ιΜ·Ής”ΟΫχ––Ζ÷ΈωΘΜ
Θ®3Θ©ΗυΨίΡΧ÷ΤΤΖΚΆ»ΥΧε÷–ΗΤΒΡ¥φ‘Ύ–Έ Ϋ≈–ΕœΘ°
Ϋβ¥π ΫβΘΚΘ®1Θ©Φλ―ιΥ° «”≤Υ°ΜΙ «»μΥ°Θ§Ω…”ΟΒΡΈο÷ « Ζ ‘μΥ°Θ°”≤Υ°÷–Φ”»κΖ ‘μΥ°≤ζ…ζ≈ίΡ≠…ΌΓΔ»μΥ°÷–Φ”»κΖ ‘μΥ°≤ζ…ζ≈ίΡ≠ΕύΘ°Θ°…ζΜν÷–Ω…”ΟΦ”»»÷σΖ–ΒΆΥ°ΒΡ”≤Ε»Θ°
Θ®2Θ©œ¥Β”ΦΝ”–»ιΜ·Ής”ΟΘ§ΡήΫΪ¥σΒΡ”ΆΒΈΖ÷…Δ≥…œΗ–ΓΒΡ”ΆΒΈΥφΥ°≥εΉΏΘ°
Θ®3Θ©‘ΎΡΧ÷ΤΤΖ÷–Μρ‘Ύ»ΥΧεΡΎΘ§ΗΤ «“‘ΈόΜζ―ΈΒΡ–Έ Ϋ¥φ‘ΎΒΡΘ§’βάοΒΡΗΤ «÷Η―Έ÷–Κ§”–ΒΡΗΤ‘ΣΥΊΘΜάœΡξ»Υ»±ΗΤΜαΜΦΙ«÷ ηΥ…Θ°
Ι ¥πΑΗΈΣΘΚ
Θ®1Θ©Ζ ‘μΥ°ΘΜΦ”»»÷σΖ–ΘΜ
Θ®2Θ©»ιΜ·
Θ®3Θ©Ι«÷ ηΥ…Θ°
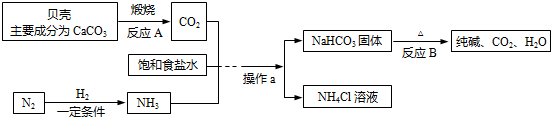
ΒψΤά ±ΨΧβΩΦ≤ιΝΥ”≤Υ°”κ»μΥ°ΒΡ÷Σ ΕΘ§Άξ≥…¥ΥΧβΘ§Ω…“‘“άΨί“―”–ΒΡ÷Σ ΕΫχ––Ϋβ¥πΘ§ τ”ΎΜυ¥Γ÷Σ ΕΘ§Ρ―Ε»÷–Β»Θ°ΡήΙΜΑ―Χβ÷––≈œΔΚΆΜ·―ß÷Σ Ε”–ΜζΒΡΫαΚœΤπά¥Θ°ΙΊΉΔ…ζΟϋΘ§Κ«ΜΛΫΓΩΒΘ§ «»Υάύ≤ΜΕœΧΫ«σΒΡ”άΚψ÷ςΧβΘ§ΥϋΦ» «…γΜα»»ΒψΘ§“≤ «÷Ί“ΣΒΡ÷–ΩΦ»»Βψ÷°“ΜΘ°


 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΫβ¥πΧβ
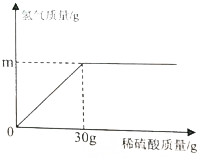 Θ®1Θ© Β―ι “œ÷”–÷ ΝΩΖ÷ ΐΈΣ98%Θ®ΟήΕ»ΘΚ1.84g/cm3Θ©ΒΡ≈®ΝρΥαΘ§”ϊΫΪ’β÷÷≈®ΝρΥαœΓ ΆΈΣ19.6%ΒΡœΓΝρΥα184gΘ§“Σ’β÷÷≈®ΝρΥαΧεΜΐΈΣmLΘΜ
Θ®1Θ© Β―ι “œ÷”–÷ ΝΩΖ÷ ΐΈΣ98%Θ®ΟήΕ»ΘΚ1.84g/cm3Θ©ΒΡ≈®ΝρΥαΘ§”ϊΫΪ’β÷÷≈®ΝρΥαœΓ ΆΈΣ19.6%ΒΡœΓΝρΥα184gΘ§“Σ’β÷÷≈®ΝρΥαΧεΜΐΈΣmLΘΜ≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΫβ¥πΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
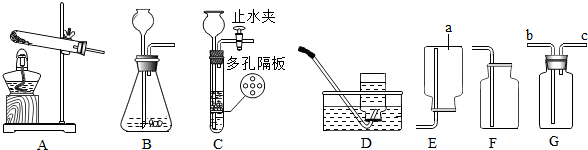
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | 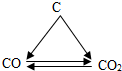 | BΘ° | 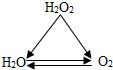 | CΘ° | 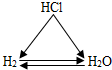 | DΘ° |  |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΧνΩ’Χβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΫβ¥πΧβ
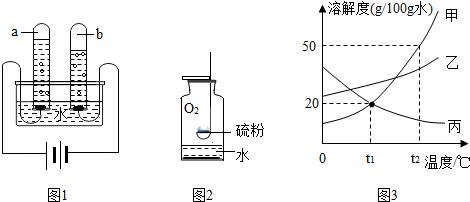
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΫβ¥πΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΧνΩ’Χβ
| Ϋπ τΒΞ÷ | Ά≠ | ¬Ν | –Ω | Χζ | «Π |
| ΒΦΒγ–‘Θ®“‘“χΒΡΒΦΒγ–‘ΈΣ100Ής±ξΉΦΘ© | 99 | 61 | 27 | 17 | 7.9 |
| ΟήΕ»/Θ®g•cm-3Θ© | 8.92 | 2.70 | 7.14 | 7.86 | 11.3 |
| »έΒψ/Γφ | 1083 | 660 | 419 | 1535 | 328 |
| ”≤Ε»Θ®“‘ΫπΗ’ ·ΒΡ”≤Ε»ΈΣ10Ής±ξΉΦΘ© | 2.5ΓΪ3 | 2ΓΪ2.9 | 2.5 | 4ΓΪ5 | 1.5 |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΧνΩ’Χβ
| Β―ιΡΩΒΡ | Β―ι…ηΦΤ | |
| A | ≥ΐ»ΞCO÷–ΒΡ…ΌΝΩH2OΚΆCO2 | œ»Ά®Ιΐ≈®ΝρΥαΘ§‘ΌΆ®Ιΐ«β―θΜ·ΡΤ»ή“Κ |
| B | ≥ΐ»ΞNaClΙΧΧε÷–ΒΡ…ΌMa2CO3 | œ»Φ”ΉψΝΩΥ°»ήΫβΘ§‘ΌΦ” œΓΝρΥαΘ§’τΖΔ |
| C | ≥ΐ»ΞKN03Θ§»ή“Κ÷–ΒΡ…ΌΝΩK2SO4 | Φ”»κ ΝΩΒΡBaΘ®N03Θ©2»ή“ΚΘ§Ιΐ¬Υ |
| D | ≥ΐ»ΞΆ≠Ζέ÷–Μλ”–ΒΡ…ΌΝΩΧζΖέ | ”Ο¥≈ΧζΈϋ“ΐ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com