


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
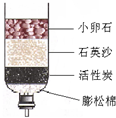 Ϊ��̽����ˮ�ľ��������̣�ijʵ��С��Ӻ���ȡ��ˮ������ʵ�飺
Ϊ��̽����ˮ�ľ��������̣�ijʵ��С��Ӻ���ȡ��ˮ������ʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡDZ���о��꼶ʮһ���¿���ѧ�Ծ��������棩 ���ͣ������
Ϊ��̽��ˮ�������̣�ijʵ��С��Ӻ�ˮ��ȡ��ˮ������ˮ�����о��á����������ˡ�����Ȳ�����
��1����ˮ�ľ������������dz����õ�����̿������Ϊ������_______�ԡ�
��2���ճ������г���_______________�ķ�����Ӳˮת��Ϊ��ˮ��
��3���ھ������ˮ��ͨ��������Cl2������ɱ���������õ�����ˮ��������ˮ��Ӧ�������ᣨHCl���ʹ�����(HClO),����������Ԫ�صĻ��ϼ�Ϊ________���÷�Ӧ�Ļ�ѧ����ʽΪ______________________��
��4���ڶ�ˮ����ʱ�����������������ö������ȣ��ڶ�����������Ԫ�صĻ��ϼ�Ϊ+4���������ȵĻ�ѧʽΪ__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com