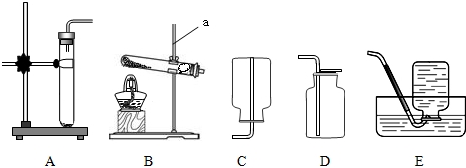
ʵ������ȡ���������װ����ͼ��ʾ����ش��������⣺
��1��д��װ��ͼ�б�����������ƣ�a
����ƿ
����ƿ
��
��2��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
����ѡ�õķ������ռ����װ��Ϊ
AD
AD
������ĸ����
��3��ʵ������װ��A��ȡ����������Cװ���ռ����������۲쵽���ܿ�������
����������
����������
�طų�ʱ�����ɽ����ռ���
��4��ѡ�������ռ�����ʱ��������������ʣ�����ɫ���ܶȢ��ܽ��Ԣܿ�ȼ�ԣ����б��뿼�ǵ���
�ڢ�
�ڢ�
������ţ���
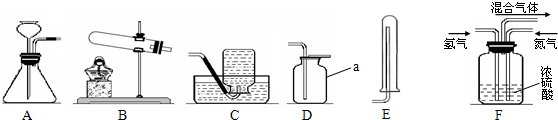
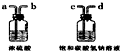
��5��Ϊ�õ���������Ķ�����̼���壬����װ�ã���ͼ���ĵ��ܰ�������������˳���ǣ�ѡ����ĸ����
A��a��b��c��d B��b��a��c��d C��c��d��a��b D��d��c��b��a
��6��������һ����ɫ���д̼�����ζ����������ˮ�����壬�������Ƶ��ʡ����ᡢҩ���Ⱦ�ϵȣ�
��ʵ���ҳ��ü����Ȼ�狀���ʯ�ҵĹ�������ķ�����ȡ��������ʵ������ȡ���ռ�����Ӧѡ���װ�������
BE
BE
�������ķ���ʽ��
2NH
4Cl+Ca��OH��
2CaCl
2+2H
2O+2NH
3��
2NH
4Cl+Ca��OH��
2CaCl
2+2H
2O+2NH
3��
�������Ϳ����������ﵽ��ը����V%����16%��25%����
����
����
���߾��緢����ը�����ж�����������֯����ˮ���ɰ�ˮ�������ܽ���֯�����ʣ�����β�������
D
D
���գ�
A��ˮ B�õ��ܲ���Ũ���� C�õ��ܲ���ϡ������ D�õ���©������ϡ������
�ڹ�ҵ���õ����������ϳɰ�����N
2+3H
22NH
3��ʵ����ģ��ϳɰ������������£�
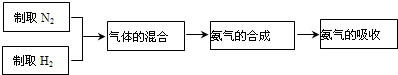
�����С�����Ļ�ϡ�����Fװ���н��еģ�Fװ�õ�������������һ�ǽ������������������ʹ������������ֻ�ϣ�����
ʹ�����͵��������ȶ�
ʹ�����͵��������ȶ�
���Ӷ���ߵ����������������ʣ�