
��ͨ���������������彻������������е��������ų�������̼��ˮ�������������ų���
������̼�����ǿ�����ԭ�еģ����������л�����ղ����أ�Ϊ��֤ʵ������⣬���˲�������ͼ��ʾ��װ�ý���ʵ�顣
(1)������ʱ��Ӧ������A____________
(����رա�����ͬ)������B________��
(2)�˺���ʱ��Ӧ������A____________������B__________����ʱ�ɹ۲쵽��ƿ�ڵ�������_____________��
(3)��ƿ����װ�Լ���������_________________________________________��
��ƿ����װ�Լ���������____________________________________________��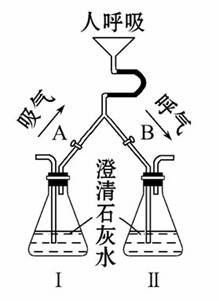 �����������������У���֤���˺����������к��еĶ�����̼�������Կ�������������Ĵ�л���
�����������������У���֤���˺����������к��еĶ�����̼�������Կ�������������Ĵ�л���
������������������Ŀ����к��������Ķ�����̼��������ʱ����������������ų�����������������л�����ղ����Ƿ��ж�����̼����������ʯ��ˮ�к���ʱ������ʹ����ʯ��ˮ����ǡ����Ϊ���ų���������ж�����̼�ĸ��ţ��轫�����еĶ�����̼�����ȥ����ʱ���˺�����������ʹ����ʯ��ˮ����ǣ���˵�����������еĶ�����̼�������л�����ղ��
�𰸣�(1)�� �ر� (2)�ر� �� ����ʯ��ˮ����� (3)��ȥ��������еĶ�����̼
��֤�˺����������к��ж�����̼



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͨ���������������彻������������е��������ų����Ƕ�����̼�����ˮ�������������ų��Ķ�����̼�ǿ����еĻ��������³´�л�IJ����أ�Ϊ��֤ʵ������⣬�������ʵ�飮
��ͨ���������������彻������������е��������ų����Ƕ�����̼�����ˮ�������������ų��Ķ�����̼�ǿ����еĻ��������³´�л�IJ����أ�Ϊ��֤ʵ������⣬�������ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͨ���������������彻��������������������ų�CO2��ˮ�������������ų��Ķ�����̼�����ǿ�����ԭ�еģ����������л�����ղ����أ�Ϊ��֤ʵ������⣬���˲�������ͼ��ʾ��װ�ý���ʵ�飺
��ͨ���������������彻��������������������ų�CO2��ˮ�������������ų��Ķ�����̼�����ǿ�����ԭ�еģ����������л�����ղ����أ�Ϊ��֤ʵ������⣬���˲�������ͼ��ʾ��װ�ý���ʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?ƽ����ģ�⣩��ͨ���������������彻������������е��������ų�������̼��ˮ������Ϊ����֤����������������������ʲô��ͬ����������ͼװ�ý���ʵ�飮
��2013?ƽ����ģ�⣩��ͨ���������������彻������������е��������ų�������̼��ˮ������Ϊ����֤����������������������ʲô��ͬ����������ͼװ�ý���ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͨ���������������彻�������Ŀ����еIJ����������ų�������̼��ˮ���������壮�������ų��Ķ�����̼���������������ԭ�еģ����������л�����ղ��Ϊ��֤ʵ������⣬���˲�������װ�ý���ʵ�飨��ͼ����
��ͨ���������������彻�������Ŀ����еIJ����������ų�������̼��ˮ���������壮�������ų��Ķ�����̼���������������ԭ�еģ����������л�����ղ��Ϊ��֤ʵ������⣬���˲�������װ�ý���ʵ�飨��ͼ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com