
���������ͷ�չ�벻����Դ����Դ��
��1������ȼ�ϵĸ��¹������£�ú��Һ��ʯ������ܵ�ú������Ȼ���� �����йؼ���ȼ�ϸ��µ����ɣ���ȷ���� (ѡ����ĸ)��
A����Ȼ�����ڿ�������Դ B������ȼ�ϱȹ���ȼ�������ʸ���
C����Ȼ����Ϊȼ�Ͽɱ�������ЧӦ�ķ��� D��ú��������ȼ���յ��˷���Դ
��2��ˮ������֮Դ���������úͱ���ˮ��Դ�������岻�ݴǵ����Ρ�
�� �跨��ȥӲˮ�е� ������ʹӲˮ��������ˮ��
�� �������������á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿��Ҫ��______���á�
��3����ˮ���д����������õĻ�ѧ��Դ�������������Ȼ�þ�ǽ���þ����Ҫ��Դ֮һ���Ӻ�ˮ����ȡ����þ���ɰ���ͼ���̽��У�
�������й�˵����ȷ���� (ѡ����ĸ)��
A�������ͨ��һ����Ӧ����ʵ�� B���������Ŀ���ǴӺ�ˮ���ᴿ�Ȼ�þ
C��������л�ѧ��ת��Ϊ���� D���ڴ��������漰�Ļ�����Ӧ������4��
���ڴ������п���ѭ�����õ������� ��
��4����������ͷ���õĴ����к�������NaCl��ijʵ��С��Ҫ�ⶨ�ô�����Na2CO3������������ȡ12g������Ʒ�����ձ��У���μ���ϡ���������ٲ������ݣ���ʱ�ձ���û�в����������ϡ����72.4g����÷�Ӧ����Һ������Ϊ80g�������ʵ��С��������¼���(д���������)��
��1�����������غ㶨�ɼ������ɶ�����̼������
��2����Ʒ��̼���Ƶ����������Ƕ���?
��1��D ��2���ơ�þ�����������Ե�Ca2+��Mg2+�� ���� ��3����B ���Ȼ��� ��4����4.4g �� 10.6g 88.3%
���������������1��A����Ȼ���ǻ�ʯȼ�ϣ����ڲ���������Դ����A����B������ȼ�ϱȹ���ȼ�Ϸ��Ӽ�����������Ӵ���֣������ʸ��ߣ���B��ȷ��C����Ȼ����Ϊȼ��Ҳ���ɶ�����̼�����Բ��ɱ�������ЧӦ�ķ�����������Ҫ�ɷּ���Ҳ�ܲ�������ЧӦ����C����D��ú��������ȼ���յ��˷���Դ����������ӹ�����D��ȷ����ѡD
��2����Ӳˮ��ָ���н϶�����Եĸơ�þ�������ˮ�������跨��ȥӲˮ�еĸơ�þ�����������Ե�Ca2+��Mg2+��������ʹӲˮ��������ˮ���ڹ������������á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿��Ҫ���������ã�
��3����A���������̼��Ƹ����������������ƣ������ƺ�ˮ��Ӧ�����������ƣ�����ͨ��һ����Ӧ����ʵ�֣�B���������Ŀ���ǴӺ�ˮ���ᴿŨ�ȸ��ߵ��Ȼ�þ��C����������ǵ���ת��Ϊ��ѧ�ܣ�D���ڴ��������漰�Ļ�����Ӧ�������η�����֪��3�֣��ֽⷴӦ�����Ϸ�Ӧ�����ֽⷴӦ��û���û���Ӧ����ѡB
�����ɵ��Ȼ�����������ˮ�γ����ᣬ�����Ժ�������þ��Ӧ�������ڴ������п���ѭ�����õ��������Ȼ��⣻
��4���ٸ��������غ㶨�ɣ����ɵĶ�����̼�������������������ٵ�����������12g+72.4g-80g�T4.4g��
������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 4.4g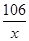 =
=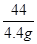 x�T10.6g
x�T10.6g
������Ʒ��̼���Ƶ���������Ϊ��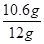 ��100%�T88.3%
��100%�T88.3%
�����ɶ�����̼������Ϊ4.4g����Ʒ��̼���Ƶ���������Ϊ88.3%
���㣺����ȼ�ϵ�ʹ������Ի�����Ӱ�죬Ӳˮ����ˮ��̼��ơ���ʯ�ҡ���ʯ��֮���ת�����εĻ�ѧ���ʣ����ݻ�ѧ��Ӧ����ʽ�ļ��㣻�Ժ�����Դ�ĺ������������ã�������Դ�����ࡢ��Դ�ķ���


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
�������˫��ˮ�Ͷ���������ȡ������ʵ�飬������ͼ1��ʾ�ij��õ�������װ�á��ش��й����⣺
��1��������Һ��������60g������������Ϊ5%��˫��ˮ��Һ����Ҫ30%��˫��ˮ��Һ g������ȡˮ�IJ����У���ˮ��ӽ�����̶�ʱ��Ӧ���� �����������ƣ���ˮ������̶ȡ�
��2������ǩ�������ƺõ���Һװ���Լ�ƿ�У�������ͼ2��ʾ�ı�ǩ�������Լ�ƿ������ǩ�����ݡ�
��3�����ݷ�Ӧԭ��ѡ��������˫��ˮ�Ͷ���������ȡ�������÷�Ӧ�Ļ�ѧ����ʽ�� ���䷢��װ�ú��ռ�װ�ÿ�ѡ����ͼ�е� ������ţ���ϡ�
��4��ʵ�鲽�裺
�ټ����������װ�õ� ��
��װҩƷ������������װ������ĩ����װ��Һ���Լ���
���ռ����壬���ô����ǵ�ľ���������������ķ����������������� �����ʡ�
�ܻ��մ����������̣�����������װ�������ڵIJ����ᆳ ��д�������ƣ�ϴ�ӡ�������ն������̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
С����С��������ͼװ�ã���̼��ƺ�ϡ����Ϊԭ������ȡ������̼���壬�����бȽϷ�����
��1��С����ͼ��ͼ���������ȡ������̼���壬��μ��������̼�Ƿ��ռ�������д����������� ��
��2��С����Ϊ�üױ��ռ�������̼����ķ�����ͼ�Ҹ��ã�����Ϊ���������ǣ� ��д��һ�㣩��
��3��ʵ�������С�췢�ַ�Ӧ�п����������о�һ���жϷ�Ӧ�����ķ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
A��B��C��D��E�������ʾ�Ϊ���л�ѧ�����漰�ij������ʣ����Ǵ�������ͼ��ת����ϵ����������ʾ�������ͷ��ָ����һ��ת�������߱�ʾ�������ʼ���Է�����Ӧ��C�dz������壬D�dz������ʣ�����Ӧ�������������ʾ�����ȥ����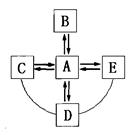
��ش��������⣺
��1����A�ڳ�����ΪҺ�壬��C�Ļ�ѧʽΪ _��
��2����A�ڳ�����Ϊ���壬��C�Ļ�ѧʽΪ ��
��3������A�ڳ�����ΪҺ�廹�����壬B�Ļ�ѧʽ��Ϊ ��D�Ļ�ѧʽ��Ϊ ��
��4��д��A��E�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������������ȷ����
| A�����õ����Ż�������ˮ���� |
| B����Ȼ��й©�������رշ��Ų�����ͨ�� |
| C��������ȼ�ŵľƾ���������������ʪ������ |
| D��ϡ��Ũ����ʱ����Ũ���Ỻ��ע��ˮ�в����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������в���ȷ���ǣ� ��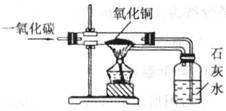
| A���÷�Ӧ��һ����̼�ǻ�ԭ�� |
| B������������Ӧ��������ͭ |
| C��ʵ��ǰҪ��ͨ��һ����̼���ٵ�ȼ�ƾ��� |
| D����װ�ÿ��Է�ֹ�ж���һ����̼����Կ�������Ⱦ��ͬʱ�ֿ��Գ��������Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������������������ϢϢ��ء�
��1������ϰ���ϳ�Ϊ����𡱵Ľ�����ͭ���������У��ѽ���______����Ԫ�ط��ţ���λ���Ƶ�������÷��Ͻ�������� ���ǿ������������ǿ������˳�����п��Ժ�ϡ���ᷢ����ѧ��Ӧ�Ľ����� ��
��2����ͼ��ʾ������Ʒ�У���Ҫ���ý��������Ե���_____����չ�Ե���______������ĸ��ţ���
��3���ӽ���ͭ�������̣�Mn���Ļ�Ϸ�ĩ�з����ij�ֽ����������һ�־��壬��Ƶ�ʵ���������¡�
����������AԪ�صĻ��ϼ�Ϊ+2����д����������ϡ����ʱ��Ӧ�Ļ�ѧ����ʽ�� ��
�����ɽ���C�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
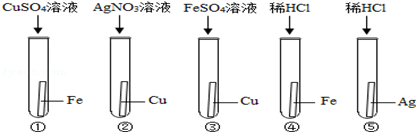
ij��ѧ��ȤС�飬Ϊ��֤Ag��Fe��Cu���ֽ����Ļ��˳���������ͼ��ʾ��ʵ��������п��Դﵽʵ��Ŀ������ǣ� ��
| A���٢� | B���ڢ� | C���ڢ� | D���ܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���ڳ�ʪ�Ŀ�����ᷢ����ʴ��֤������һ���μ��˷�Ӧ����Ҫ����ʵ����( )
| A���٢� | B���٢� | C���ڢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com