



 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
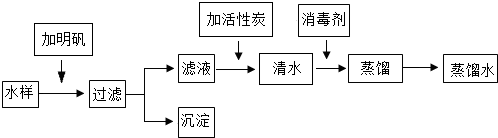
 С��ͬѧȥ����ʱ����ƿװ��һЩɽ�µ�Ȫˮ������ʵ���ң�����ʦ��ָ���£����������̽���ʵ�飬��ȡ����ˮ��
С��ͬѧȥ����ʱ����ƿװ��һЩɽ�µ�Ȫˮ������ʵ���ң�����ʦ��ָ���£����������̽���ʵ�飬��ȡ����ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 С��ͬѧȥ����ʱ����ƿװ��һЩɽ�µ�Ȫˮ������ʵ���ң�����ʦ��ָ���£����������̽���ʵ�飬��ȡ����ˮ��
С��ͬѧȥ����ʱ����ƿװ��һЩɽ�µ�Ȫˮ������ʵ���ң�����ʦ��ָ���£����������̽���ʵ�飬��ȡ����ˮ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��Ŀ | �� |
| �й�ָ�� | ����ζ������� |
| ��ѧָ�� | pH6.5--8.5��ͭ��1.0mg?L-1������0.3mg?L-1�������1.0mg?L-1�������ȡ�0.3mg?L-1 |
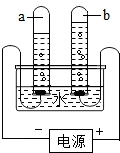
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com