£Ø2013?¶«ĄöĒų¶žÄ££©øł¾ŻĻĀĮŠ×°ÖĆĶ¼£¬»Ų“šÓŠ¹ŲĪŹĢā£ŗ
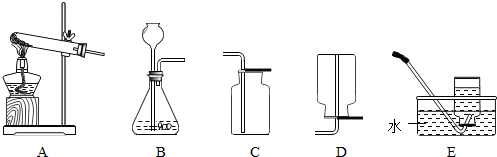
£Ø1£©ĄūÓĆĶ¼ÖŠA”¢C×°ÖƵÄ×éŗĻæÉŅŌÖĘČ”Ä³ÖÖĘųĢ壬ŹµŃéŹŅÖĘČ”øĆĘųĢåµÄ»Æѧ·½³ĢŹ½ŹĒ
£¬ŃéĀś·½·ØŹĒ
½«“ų»šŠĒµÄľĢõææ½ü¼ÆĘųĘææŚ£¬ČōľĢõŃøĖŁø“Č¼£¬Ö¤Ć÷ŅŃŹÕ¼ÆĀśŃõĘų
½«“ų»šŠĒµÄľĢõææ½ü¼ÆĘųĘææŚ£¬ČōľĢõŃøĖŁø“Č¼£¬Ö¤Ć÷ŅŃŹÕ¼ÆĀśŃõĘų
£®
£Ø2£©ŹµŃéŹŅĶس£ĄūÓĆĶ¼ÖŠ
BC
BC
×°ÖƵÄ×éŗĻÖĘČ”¶žŃõ»ÆĢ¼£ØŃ”Ģī×ÖÄø£©£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
CaCO3+2HClØTCaCl2+CO2”ü+H2O
CaCO3+2HClØTCaCl2+CO2”ü+H2O
£®
¢ŁĪŖĮĖµĆµ½“æ¾»”¢øÉŌļµÄ¶žŃõ»ÆĢ¼ĘųĢ壬³żŌÓ×°ÖĆ£ØČēĶ¼£©µÄµ¼¹Ü°“ĘųĮ÷·½ĻņĮ¬½ÓĖ³ŠņŹĒ
C
C
£ØŃ”Ģī×ÖÄø£©£®
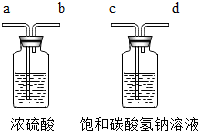
A£®a”śb”śc”śd B£®b”śa”śc”śd
C£®c”śd”śa”śb D£®d”śc”śb”śa
¢Ś³£ÓĆ³ĪĒåŹÆ»ŅĖ®¼ģŃ鶞Ńõ»ÆĢ¼µÄ“ęŌŚ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
CO2+Ca£ØOH£©2ØTCaCO3”ż+H2O
CO2+Ca£ØOH£©2ØTCaCO3”ż+H2O
£»Čō¶žŃõ»ÆĢ¼¹żĮ棬¶žŃõ»ÆĢ¼ÓÖ»įÓėĢ¼ĖįøĘ”¢Ė®·“Ӧɜ³ÉŅ×ČܵÄĢ¼ĖįĒāøĘ[Ca£ØHCO
3£©
2]£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
CO2+CaCO3+H2OØTCa£ØHCO3£©2
CO2+CaCO3+H2OØTCa£ØHCO3£©2
£®Ļņŗ¬ÓŠĒāŃõ»ÆøĘ14.8gµÄŹÆ»ŅĖ®Ąļ»ŗ»ŗĶØČėŅ»¶ØĮ涞Ńõ»ÆĢ¼£¬³ä·Ö·“Ó¦ŗóČōÉś³É10g³Įµķ£¬ŌņĶØČė¶žŃõ»ÆĢ¼µÄÖŹĮææÉÄÜĪŖ
AC
AC
£ØŃ”Ģī×ÖÄø£©£®
A£®4.4g B£®8.8g C£®13.2g D£®17.6g£®






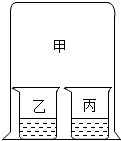 £Ø2013?¶«ĄöĒų¶žÄ££©ČēĶ¼ĖłŹ¾£¬ŌŚŠ”ÉÕ±ŅŅŗĶ±ūÄŚ·Ö±š·ÅČė²»Ķ¬µÄĪļÖŹŗó£¬Į¢¼“ÓĆ“óÉÕ±¼×ÕÖ×”Š”ÉÕ±ŅŅŗĶ±ū£®ĻĀĮŠÓŠ¹ŲŹµŃéĻÖĻó²»ÕżČ·µÄŹĒ£Ø””””£©
£Ø2013?¶«ĄöĒų¶žÄ££©ČēĶ¼ĖłŹ¾£¬ŌŚŠ”ÉÕ±ŅŅŗĶ±ūÄŚ·Ö±š·ÅČė²»Ķ¬µÄĪļÖŹŗó£¬Į¢¼“ÓĆ“óÉÕ±¼×ÕÖ×”Š”ÉÕ±ŅŅŗĶ±ū£®ĻĀĮŠÓŠ¹ŲŹµŃéĻÖĻó²»ÕżČ·µÄŹĒ£Ø””””£©
 £Ø2013?¶«ĄöĒų¶žÄ££©ČēĶ¼ŹĒČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻߣ®
£Ø2013?¶«ĄöĒų¶žÄ££©ČēĶ¼ŹĒČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻߣ®