
����һƿδ������Ũ���ᣬ�Լ�ƿ��ǩ�ϵIJ���������ͼ��ʾ��

(1)H2SO4����Է���������________��
(2)H2SO4�����У�H��S��O��ԭ�Ӹ�����Ϊ________��
(3)��ƿŨ���������Ϊ________g��
(4)��100 g98���ĸ�Ũ����ϡ�ͳ�19.6����ϡ���ᣬ��Ҫˮ����g��
(5)ijͬѧ��п����19.6����ϡ���ᷴӦ��ȡ��������Ӧ���õ�ϡ��������������Ĺ�ϵ����ͼ��
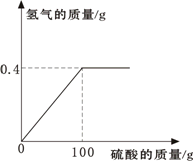
���㣺
��������Ҫ����g��п����
����ȫ��Ӧ��������Һ��������������Ϊ���٣�(����һλС����)
 ȫ��������ϵ�д�
ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijУ��ѧ����С���ͬѧ��ҪһЩ��������ѧʵ�飮����һƿδ������Ũ���ᣬ��ǩ��ͼ�����Ķ���ǩ�ϵ�˵�����ش��������⣮
ijУ��ѧ����С���ͬѧ��ҪһЩ��������ѧʵ�飮����һƿδ������Ũ���ᣬ��ǩ��ͼ�����Ķ���ǩ�ϵ�˵�����ش��������⣮| ʵ����� | 1 | 2 | 3 |
| �Ͻ�������g�� | 10 | 10 | 10 |
| ϡ����������g�� | 25 | 50 | 75 |
| ����������������g�� | 0.1 | 0.2 | 0.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ����һƿδ������Ũ���ᣬ�Լ�ƿ��ǩ�ϵIJ���������ͼ��ʾ��������й���Ϣ�ش��㣺
����һƿδ������Ũ���ᣬ�Լ�ƿ��ǩ�ϵIJ���������ͼ��ʾ��������й���Ϣ�ش��㣺| ���ᣨ500mL�� Ʒ �� ���� ��ѧʽ H2SO4 ��Է�������98 �� �� 1.84g/cm3 ��������98% ��1�������� �� �� Ԫ�ء��� �� Ԫ�ء��� �� Ԫ����ɣ�������Ԫ�أ������� ���� ���ǡ����ǣ������������Է�������Ϊ98 98 ������Ϊ���������Ậ��Ԫ��������ȵ������������������������ ����������������� ����2�����Լ�ƿ��������Һ�������� 920 920 g����3��������ϯ������20g��Ũ��������20%��ϡ���ᣬ����ϴ������������⣮�������Ƹ���Һʱ����98%��Ũ������ˮ��������Ӧ��Ϊ 10��39 10��39 ����4��ij�����������Ȼ��ƺ��Ȼ�����ɣ�ȡ32.8g�ù���������ȫ����ˮ������μ�������20%��ϡ���ᣬ�������������������ϡ�������������ͼ��ʾ��ϵ������32.8g�����������Ȼ��ƺ��Ȼ����������� ��5���������ϡ����պ�ʹ�����������ʱ�������û������ˡ�ϴ�ӣ�����Һ������ϡ����200g����������ĺ��Բ��ƣ����������Һ�����ʵ���������Ϊ �Ȼ��Ƶ���������Ϊ6%���Ȼ������������Ϊ3.65% �Ȼ��Ƶ���������Ϊ6%���Ȼ������������Ϊ3.65% ��������Һ�к��ж������ʣ���ÿ�����ʵ���������Ϊ�����ʵ���������Һ������֮�ȣ�[ע����3������4��������д���������]����6��ϲ��̽��������˼������������������Ŀ����ң���30g��Fe��Al��Mg��Zn�Ľ�����ĩͶ��������ϡ������ȫ���ܽ��꣬�ٽ���Һ���ɵ���ˮ�������126g����ͬʱ��������������Ϊ 2 2 g��̽�����������֣�����ͬŨ�ȵ����ᣬ��һƿδ���ʡ����ֱ��ʡ�ȫ���ʣ����ʶ����������ƣ�����ʯ����Ʒ��Ӧ����Ҫ��������������ȣ���ԭ���������ƺ�̼��ƶ���ÿ40g��Ԫ������136g����ƣ�����98g���� �����ƺ�̼��ƶ���ÿ40g��Ԫ������136g����ƣ�����98g���� ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ������
|
| ʵ����� | 1 | 2 | 3 |
| �Ͻ�������g�� | 10 | 10 | 10 |
| ϡ����������g�� | 25 | 50 | 75 |
| ����������������g�� | 0.1 | 0.2 | 0.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һƿδ������Ũ���ᣬ�Լ�ƿ��ǩ�ϵIJ���������ͼ��ʾ��������й���Ϣ���㣺
�Ÿ��Լ���������Һ�������� �� g������С���ø�Ũ��������20����ϡ���ᣬ����ϴ������������⡣�������Ƹ���Һʱ����98����Ũ������ˮ��������Ӧ��Ϊ �� ��
��ij�����������Ȼ��ƺ��Ȼ��������ɣ�ȡ32.8g�ù���������ȫ����ˮ������μ�������20����ϡ�������������������ϡ���������������ͼ��ʾ��ϵ������32.8g�����������Ȼ������Ȼ��Ƶ�������
���������ϡ����պ�ʹ���������ʱ�������û������ˡ�ϴ�ӣ�����Һ200g����������ĺ��Բ��ƣ����������Һ������HCl������������������Һ�к��ж������ʣ���ij���ʵ���������Ϊ�����ʵ���������Һ��
����֮�ȣ�����Ӧ�ķ�����Ϊ��BaCl2 + H2SO4 = BaSO4�� + 2HCl��


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com