
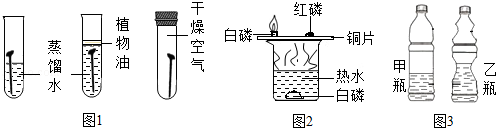


 ĘߊĒĶ¼ŹéæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ĘߊĒĶ¼ŹéæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
| ŹµŃé²Ł×÷ | ŹµŃéĻÖĻó |
| ȔɣĮæѳʷӌŹŌ¹ÜÖŠ£¬ĀżĀżµĪ¼ÓBaCl2ČÜŅŗ | ³öĻÖ°×É«³Įµķ |
| ¾²ÖĆŅ»¶ĪŹ±¼äŗó£¬ĒćČ„ÉĻ²ćĒåŅŗ£¬Ļņ³ĮµķÖŠµĪ¼ÓĻ”ŃĪĖį | °×É«³ĮµķČ«²æČܽā£¬²¢²śÉś“óĮæĘųÅŻ |
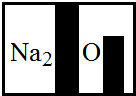
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
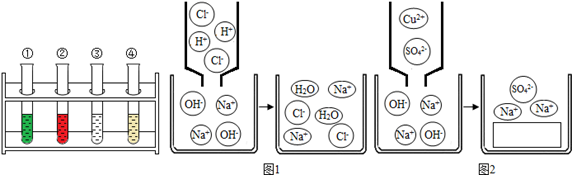
| | NaOHČÜŅŗ | Ļ”ŃĪĖį | Ę»¹ūÖ |
| ×Ļ°ü²ĖÖŅŗ | ¢ŁĀĢÉ« | ¢ŚŗģÉ« | ŗģÉ« |
| °×²ĖÖŅŗ | ¢ŪĪŽÉ« | ¢Üµ»ĘÉ« | µ»ĘÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
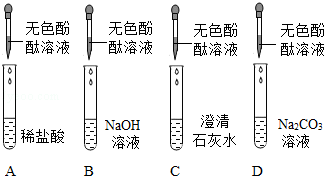
| ŹµŃé²Ł×÷ | ĻÖĻóÓė½įĀŪ |
| | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
| ŹµŃéÄŚČŻ | ĻÖĻó | ½įĀŪ |
| £Ø1£©·Ö±š½«ÉŁĮæÓÅÖŹÕäÖé·ŪŗĶĮ®¼ŪÕäÖé·Ū·ÅČėŹŌ¹ÜÖŠ£¬¼ÓÉŁĮæĖ®£¬¾²ÖĆŅ»¶ĪŹ±¼äŗ󣬵Ī¼Ó”” ””£® | Į®¼ŪÕäÖé·ŪµÄÉĻ²ćĒåŅŗ±äŗģ£¬ÓÅÖŹÕäÖé·ŪµÄČÜŅŗƻӊ±äÉ« | ²ĀĻė¢Ł³ÉĮ¢ |
| £Ø2£©·Ö±š½«ÉŁĮæÓÅÖŹÕäÖé·ŪŗĶĮ®¼ŪÕäÖé·Ū·ÅČėŹŌ¹ÜÖŠ£¬¼ÓÉŁĮæĖ®£¬¼ÓČČ£® | ÓÅÖŹÕäÖé·ŪµÄČÜŅŗÓŠ»ĘÉ«³öĻÖ£¬¾Ö²æ±äŗŚ£¬Į®¼ŪÕäÖé·ŪµÄČÜŅŗƻӊĆ÷ĻŌĻÖĻó | ²ĀĻė¢Ś³ÉĮ¢ |
| | ÓÅÖŹÕäÖé·Ū | Į®¼ŪÕäÖé·Ū |
| ÕäÖé·ŪµÄÖŹĮæ | 100 g | 100 g |
| ¼ÓČėŃĪĖįµÄÖŹĮæ | 460.0 g | 501.3 g |
| ÉÕ±ÖŠ×īÖÕĪļÖŹµÄ×ÜÖŹĮæ | 520.0 g | 557.7 g |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
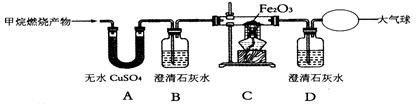
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
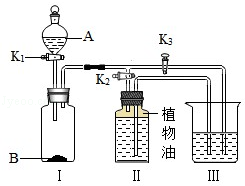
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com