

 =90mL£»
=90mL£» ×100%=26.5%
×100%=26.5% 


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø4£©Š”Ć÷Ķ¬Ń§½«³ĘĮæĶź±ĻµÄŹ³ŃĪ×ŖŅʵ½ÉÕ±ĄļŹ±£¬²»É÷½«ÉŁĮæŹ³ŃĪČ÷ĀäŌŚ×ĄĆęÉĻ£¬ÕāŃł»įŹ¹ĖłÅäÖʵÄČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹż
£Ø4£©Š”Ć÷Ķ¬Ń§½«³ĘĮæĶź±ĻµÄŹ³ŃĪ×ŖŅʵ½ÉÕ±ĄļŹ±£¬²»É÷½«ÉŁĮæŹ³ŃĪČ÷ĀäŌŚ×ĄĆęÉĻ£¬ÕāŃł»įŹ¹ĖłÅäÖʵÄČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹż²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
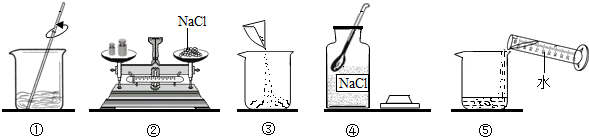
(7·Ö)ĻĀĮŠŹĒŠ”Ć÷Ķ¬Ń§ÅäÖĘ100 g 10% NaClČÜŅŗµÄŹµŃé²Ł×÷Ź¾ŅāĶ¼”£

(1)øĆŹµŃéÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ____________(ĢīŠņŗÅ)”£
(2)ČĻÕę¹Ū²ģÖø³öĶ¼ÖŠ“ķĪóµÄ²Ł×÷²½Öč________(ĢīŠņŗÅ)”£
(3)ÅäÖĘøĆČÜŅŗŠčŅŖNaCl¹ĢĢå________g£¬ŠčŅŖĖ®________mL(¦ŃĖ®£½1 g/mL)”£

(4)Š”Ć÷Ķ¬Ń§½«³ĘĮæĶź±ĻµÄŹ³ŃĪ×ŖŅʵ½ÉÕ±ĄļŹ±£¬²»É÷½«ÉŁĮæŹ³ŃĪČ÷ĀäŌŚ×ĄĆęÉĻ£¬ÕāŃł»įŹ¹ĖłÅäÖʵÄČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹż________10%(Ģī”°£¾”±”°£½”±»ņ”°£¼”±)”£
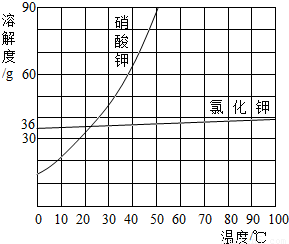
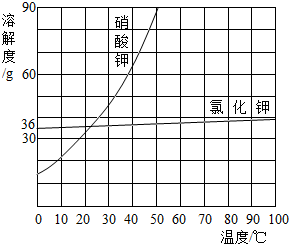
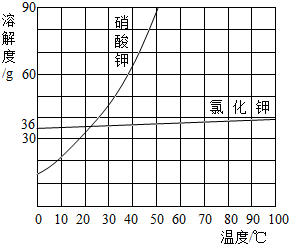
(5)²æ·ÖĪļÖŹČܽā¶ČĒśĻßČēÓŅĶ¼ĖłŹ¾£¬ŌŚ20 ”ꏱ£¬½«40 gNaCl¹ĢĢå¼ÓČėµ½100 gĖ®ÖŠ£¬½Į°čŹ¹Ęä³ä·ÖČܽā£¬ÄćČĻĪŖĖłµĆNaClČÜŅŗµÄÖŹĮæŹĒ________g£¬ČÜÖŹÖŹĮæ·ÖŹżŹĒ________%(¾«Č·µ½0.1%)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £Ø4£©Š”Ć÷Ķ¬Ń§½«³ĘĮæĶź±ĻµÄŹ³ŃĪ×ŖŅʵ½ÉÕ±ĄļŹ±£¬²»É÷½«ÉŁĮæŹ³ŃĪČ÷ĀäŌŚ×ĄĆęÉĻ£¬ÕāŃł»įŹ¹ĖłÅäÖʵÄČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹż______10%£ØĢī”°£¾”±”°=”±»ņ”°£¼”±£©£®
£Ø4£©Š”Ć÷Ķ¬Ń§½«³ĘĮæĶź±ĻµÄŹ³ŃĪ×ŖŅʵ½ÉÕ±ĄļŹ±£¬²»É÷½«ÉŁĮæŹ³ŃĪČ÷ĀäŌŚ×ĄĆęÉĻ£¬ÕāŃł»įŹ¹ĖłÅäÖʵÄČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹż______10%£ØĢī”°£¾”±”°=”±»ņ”°£¼”±£©£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com