��2013?�綫��ģ�⣩���龳-����-����-���á��ǿ�չ�о���ѧϰ��һ��˼·��
���龳��ϰ�����и������ʼ���ܹ�������Ӧ����д���ۡ��ܷ�Ӧ�Ļ�ѧ����ʽ��
������ͭ��Һ������������Һ ��̼������Һ������
������������Һ������
H2SO4+Ba��OH��2�TBaSO4��+2H2O
H2SO4+Ba��OH��2�TBaSO4��+2H2O
��
��̼������Һ�����ᱵ��Һ
Na2CO3+Ba��NO3��2=2NaNO3+BaCO3��
Na2CO3+Ba��NO3��2=2NaNO3+BaCO3��
��
���о����ۡ�������ӦΪʲô�ܹ�����������Ϊ��Щ��Ӧ��������������������������������ɳ����������ˮ�������Cu
2+��OH
-�������������ͭ����������H
+��CO
32-������ɶ�����̼�����ˮ������
�����Ӻ���������ӽ�ϳ����ᱵ�����������Ӻ����������ӽ�ϳ�ˮ
�����Ӻ���������ӽ�ϳ����ᱵ�����������Ӻ����������ӽ�ϳ�ˮ
��
����
̼������Ӻͱ����ӽ�ϳ�̼�ᱵ����
̼������Ӻͱ����ӽ�ϳ�̼�ᱵ����
��
���������硿����һ����˳��������Щ���ӣ��Ϳ����γ�һ��������ʽ���������У��ö�����������Щ����������������������ɳ����������ˮ������CO
32-��SO
42-���뽫��������������ʵġ������������У�ʹ���γ�һ����Ϊ�����ĸ��ֽⷴӦ���磮
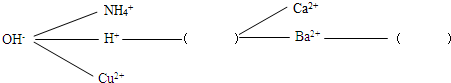
����չ���á�ͨ���γɵ����磬�����жϻ�������ܷ棬�������ʵļ����������ʵij��ӵȣ�������������Һ�к�������������ͭ���ʣ����Լ���
NaOH
NaOH
����д��ѧʽ��ȥ����





