
 ��ͼ��ij�����Լ�ƿ��ǩ�ϵIJ���˵����
��ͼ��ij�����Լ�ƿ��ǩ�ϵIJ���˵�������� ��1�������ṩ�����ݿ��Խ�����ط���ļ��㣻
��2��������Ͳ��ȡˮʱ���Ӷ������ᵼ����ȡˮ�����ƫ�Ӷ�����������Һ����������ƫС��
�����ձ��м�ˮʱ��ˮ�������ᵼ���ܼ�����ƫС���Ӷ�����������Һ����������ƫ��
�۳������в��������ʵ�ʳ����������Һ���ᵼ���Ȼ�������ƫС���Ӷ�����������Һ����������ƫС��
�ܽ����úõ���Һ���Լ�ƿ��ת��ʱ����������ʱ����Ӱ��������Һ������������
��3��Ũ��ˮ��Ũ���ᶼ�ӷ����������Ȼ��ⷴӦ���ɰ�ɫ�����Ȼ�泥�
��� �⣺��1��ȡ10mL ����������Ƴ� 5% �����������Ϊ����10mL��1.18g/mL��36%����5%=84.96g��
���84.96��
��2��������Ͳ��ȡˮʱ���Ӷ������ᵼ����ȡˮ�����ƫ�Ӷ�����������Һ����������ƫС��
�����ձ��м�ˮʱ��ˮ�������ᵼ���ܼ�����ƫС���Ӷ�����������Һ����������ƫ��
�۳������в��������ʵ�ʳ����������Һ���ᵼ���Ȼ�������ƫС���Ӷ�����������Һ����������ƫС��
�ܽ����úõ���Һ���Լ�ƿ��ת��ʱ����������ʱ����Ӱ��������Һ������������
����٢ۣ�
��3����ƿ��ʱ����պ��Ũ��ˮ�IJ���������ƿ�ڣ�������������̣�������ΪŨ��ˮ��Ũ���ᶼ���лӷ��ԣ����߽Ӵ��ᷴӦ�����Ȼ�粒���С������
���Ũ��ˮ��Ũ���ᶼ���лӷ��ԣ����߽Ӵ��ᷴӦ�����Ȼ�粒���С������
���� �������ʵ�飬��ѧ�ؽ���ʵ�顢����ʵ�飬�ǵó���ȷʵ����۵�ǰ�ᣬ���Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊѧ�û�ѧ֪ʶ�춨������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | X�Ļ�ѧʽΪH2O | B�� | �÷�Ӧ�����û���Ӧ | ||
| C�� | ��Ӧǰ����������ʷ����ı� | D�� | ��Ӧ���������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
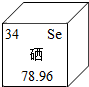
| A�� | ��ԭ�Ӻ���������Ϊ34 | B�� | �������ԭ��������78.96g | ||
| C�� | �����ڽ���Ԫ�� | D�� | ��ԭ�Ӻ���������Ϊ34 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ӧ��ȡ��ˮ�����Ϊ180mL | |
| B�� | ʵ������Ҫ�����������ձ�����Ͳ�������� | |
| C�� | װƿʱ��С����©һ������Һ����ƿ��������ҺŨ�ȵ���10% | |
| D�� | �����ڱڸ���ˮ����ձ�������Һ����������Һ����������С��10% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ͼΪij��ʯ��Ʒ1�����У�����Ԫ�غ����ı���ͼ������Ʒ�в����ܺ������к��ֻ������������
ͼΪij��ʯ��Ʒ1�����У�����Ԫ�غ����ı���ͼ������Ʒ�в����ܺ������к��ֻ������������| A�� | ̼��� | B�� | ����� | C�� | ������ | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ҩ Ʒ | �� �� �� Χ |
| �������� | ��Ҫ����ҽԺ�����������������н�ǿ��ʴ�Ժ�Ư���ԣ���Ƥ����ǿ�Ҵ̼��� |
| ������� | 0.1%����Һ�����ڹϹ����߲˺�ʳ�ߵ�������1%����Һ���ڳ�ϴ����ҧ�˵��˿ڣ� |
| ˫��ˮ | Ư��ɱ������ǿ��3%��˫��ˮ�����ڴ��˻�ҩ���������������£���Ͽ�ֽ⣮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com