
(2007
��������19)ͼʾΪ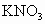 ��
�� (��������)��NaCl���ܽ�����ߣ������ͼ��ش��������⣺
(��������)��NaCl���ܽ�����ߣ������ͼ��ش��������⣺

(1)60
��ʱ�� ������Һ�����ʵ���������________(����ڡ������ڡ���С�ڡ�)
������Һ�����ʵ���������________(����ڡ������ڡ���С�ڡ�) ������Һ�����ʵ�����������
������Һ�����ʵ�����������
(2)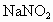
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
(2007
��������16)����ũ�Ƴ�ʹ�õ�ij����������Ӫ��Һ�к�������أ�����������ֽ⣬�����ķ�Ӧ�ǣ�


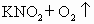
(1)
�����淽�������ϻ�ѧ��������(2)
(3)
����������ԭ�ӹ��ɣ���ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ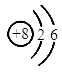 �����������________�����ӣ�
�����������________�����ӣ�
(4)
���� �������Ʋ⣬�����淽��ӦΪ________________��
�������Ʋ⣬�����淽��ӦΪ________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
(2007
��������20)��ͼ��ʵ������һ����̼��ԭ��������ʵ��װ��ͼ��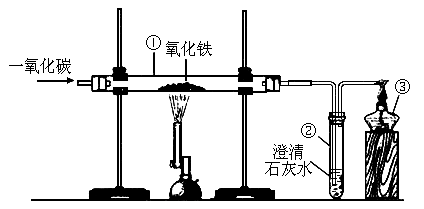
�Իش�
(1)
�����۵�������_____________��(2)
ʵ��������������е�������________________________________���������з�����Ӧ�Ļ�ѧ����ʽ��_______________________________��(3)
ʵ������е�β������ֱ����������е�ԭ����_______________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
(2007
��������22)��ѧ����С���ͬѧ��ʵ���Ҳⶨij��Ƴ����� ��Һ�����ʵ�����������ȡ��
��Һ�����ʵ�����������ȡ�� ��Һ50g�����м���ijδ֪��������������NaOH��Һ40g������ǡ����ȫ��Ӧ������
��Һ50g�����м���ijδ֪��������������NaOH��Һ40g������ǡ����ȫ��Ӧ������ ����4.9g��
����4.9g��
(1)
���� ��Һ�����ʵ�����������
��Һ�����ʵ�����������
(2)
���������غ㶨�ɷ�������Ӧ��������Һ������=________g���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com