
��ͼ��ʾΪʵ�����г�����������Ʊ������Ӻ�����ʵ��IJ����������Ը�����ĿҪ�ش��������⣺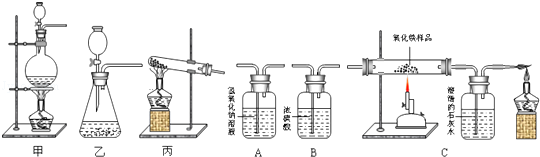
��1����д������װ�����Ʊ���һ������Ļ�ѧ����ʽ�� ������Ҫд�����װ���������Եķ����� ����2��С��ͬѧ���о�һ����̼�Ļ�ԭ�ԣ�ͨ����������֪�������ᣨH2C2O4����Ũ�����ϼ��Ȼ����һ����̼����Ӧ����ʽΪH2C2O4=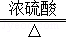 CO��+CO2��+H2O
CO��+CO2��+H2O
���ò�����ȡCO������ΪӦѡ��ķ���װ���� ������ס������ҡ���������
��Ҫ�ø��������һ����̼��ԭ���������ⶨ��������������ʣ����С��������װ�ý������ӣ�����������˳���ǣ��� ����������Ⱥ�������������� ��
����֤��CO������л�ԭ�Ե�ʵ���������� ����
��С���������12����������Ʒ�����������������������ռ��������������ݣ����������ܷ����ķ�Ӧ����Ӧ��ȫ����
����һ�����ݲμӷ�Ӧ�IJ�������������������һ����̼5.6�ˣ�
���ݶ�����Ӧǰ�����ʯ��ˮ����6.6�ˣ�
��ѡ�������һ�����ݣ������12����������Ʒ������������������Ϊ�� �������ս����ȷ��0.1%����
��1��CaCO3+2HCl=CaCl2+CO2��+H2O���������ɣ����رտ��غ��Ȱѵ��ܵ�һ�˲���ˮ�У�Ȼ�����ֽ�����ƿ����ڣ��۲쵼�ܿ��Ƿ�������ð����
��2���� �� �ڼס�A��B��C��һ����̼��������̼��
��C�к�ɫ���ɫ������ʯ��ˮ����ǡ�
��66.7%��
���������������1��ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ����װ���������Եķ����ǣ��رտ��غ��Ȱѵ��ܵ�һ�˲���ˮ�У�Ȼ�����ֽ�����ƿ����ڣ��۲쵼�ܿ��Ƿ�������ð����
��2���ٲ��ᣨH2C2O4����Ũ�����ϼ��Ȼ����һ����̼�������Ҫ���ȣ��ʴ�Ϊ�ס�
�ڲ��ᣨH2C2O4����Ũ�����ϼ�������һ����̼�Ͷ�����̼��ˮ����˲�����Ⱥ��������������һ����̼�Ͷ�����̼��Ҫ�õ����������һ����̼��Ӧ���ȳ����ʶ�����̼��Ȼ���ٸ���ʴ�Ϊ���ס�A��B��C��һ����̼��������̼��
����֤��CO������л�ԭ�Ե�ʵ�������ǣ�C�к�ɫ���ɫ������ʯ��ˮ����ǡ�
������һ�����У�����һ����̼������������������������ʱ�������ƫ����Ϊ����������Ӧ��һ����̼С��5.6g�����Ӧ�������ݶ��е����ݼ��㣻��Ӧǰ�����ʯ��ˮ����6.6�ˣ���������̼��������6.6g��Ȼ����ݶ�����̼���������������������������������������������
������������������Ϊx
3CO+Fe2O3 2Fe+3CO2
2Fe+3CO2
160 3��44
12x 6.6g 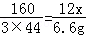 x��66.7%
x��66.7%
���㣺���⿼�鳣������ķ���װ�ú��ռ�װ����ѡȡ���������װ�õ������ԣ���������ļ�������ӷ�����һ����̼�Ļ�ѧ���ʣ����ݻ�ѧ��Ӧ����ʽ�ļ��㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͨ��һ��Ļ�ѧѧϰ�����Ѿ�������ʵ������ȡ������йع��ɣ���������ʦ�ṩ��һЩʵ��װ�ã�������ͼ�ش��������⣺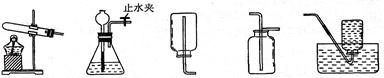
A B C D E
(1)�ر�Bװ���е�ֹˮ�кӳ���©������ƿ��ע��һ������ˮ����ֹ����ͼ��ʾ����Bװ���Ƿ�©����_____ ___(�©����������©��������ȷ����)��
(2)ѡ��Eװ���ռ��������ռ�����������������ԭ������� (д��һ��)��
(3)С��ͬѧ�ü����Ȼ�狀��������ƵĹ�����������ȡ��������Ӧѡ��ķ���װ����__ __�����ռ�������װ��ֻ����C���ɴ˿�֪���������е������� (д��һ��)��
(4)NH3��һ�ּ������壬����ʱ����ѡ�����и�����е� (�����)��
A�������������� B��Ũ���� C����ʯ��
(5)С��ͬѧ���ռ��������ļ���ƿ�����ڵ�����ɫ��̪��ˮ�У��۲쵽�������Ǽ���ƿ�ڵ�ˮ������������ˮ��____ ____ (��һ����ɫ) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ�ش����⡣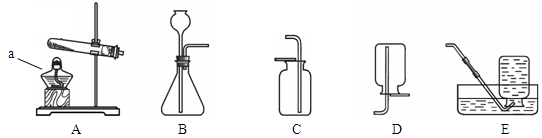
��1������a�������� ��
��2��ʵ������ȡ������̼��ѡ�õķ���װ���� ����װ����ţ���ͬ������ѡ��װ��E�ռ�������̼��ԭ���� ��
��3��ʵ�����ø��������ȡ������Ӧ�Ļ�ѧ����ʽ�� _ ����ѡ�õ��ռ�װ����E�� ����Ҫ��ʵ�鲽���У��ټ��� ��װҩƷ���̶�
�Թ� �ۼ��װ�õ������� ������ˮ���ռ����� ��ֹͣ���� �����ܴ�ˮ
����ȡ������ȷ�IJ���˳���� _ ������ű�ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʵ���ҳ��������Ľ�װ�ã�ʡ�Թ̶�װ�ã�������Ҫ��ش�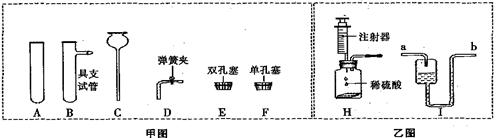
��1����ͼ������C������Ϊ______________��
��2��ʵ�����ø��������ȡ��������ѧ����ʽ��____________________________���䷢��װ�ó��õ���ͼ�����⣬����Ҫ��������_______�����̶�װ�á������⣩��
��3��ʵ������п��ϡ������ȡ��������ѧ����ʽ��_____________________��������װ�ò�����ͼH��������ϵ��ŵ���______________________________�������ռ��ķ�����____________����װ����H�������Ƶ�װ�ã�Ӧ�Ӽ�ͼ��ѡ�� __________������ĸ��ţ���
��4������ҽ����Һװ�ã����ڹ۲��Һ��������������Ϊ���������ʵ���װ�ã������ʵ�����Ӧ��___��� a ����B�������룬I��Һ�岻��װ����ԭ����_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������װ��ͼ�ش����⡣
�������a�������� ������b������ ��
��ʵ����������غͶ���������ȡ���ռ��ϴ���������Ӧ���õ�װ������� ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
����ͼ�ķ���װ���з������շ�����ԭ���ļ���װ���� ������ĸ���ţ���
�������⣨H2S����һ���ж������壬���ܶȱȿ����Ĵ�������ˮ�γ������ᡣʵ����ͨ�������շ�������������շ�������ȡ�������壬˵��ʵ������ȡ��������ķ�Ӧ��״̬�ͷ�Ӧ������ ������������Ϣʵ������ȡ�������������ռ�װ���� ������ĸ���ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ��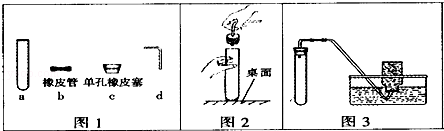
��1������ͼ1�ش�д��a�������� ����
��2������ͼ2��ʾ����������ɵĺ��֮һ���� ����
��3������ͼ3װ�ã��г�װ��δ�������ܽ��е�ʵ������ ��
| A���ø������������ | B����ʯ��ʯ��ϡ�����ƶ�����̼ |
| C����п��ϡ���������� | D����˫��ˮ��������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ʵ������ȡ����IJ���װ�ã�������ѧ��ѧ֪ʶ���ش��й����⣺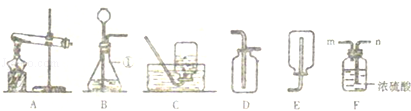
��1��д��ͼ�б�Ţ������������� ����
��2��ʵ�����ø��������ȡ������������װ��ѡ��A�����װ��A��������һ��Ķ����� ������д���÷�Ӧ�Ļ�ѧ����ʽ�� ����
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽ���� ������ȡ��������Ӧѡ��ķ���װ������ ��������ĸ����ѡ��÷���װ�õ��������� ������Ҫ�ռ�������Ķ�����̼���壬Ӧ������װ����Fװ���е��� �����m����n��������������Dװ���ռ�������̼�������ķ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ�����ﳣ������װ����ȡ���壮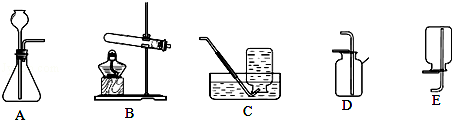
��1���ø�������������Ļ�ѧ����ʽΪ�� ����Ӧѡ��ķ���װ���ǣ���װ����ĸ����ͬ���� ����
��2���ù��������������Ļ�ѧ����ʽΪ�� ����Ӧѡ��ķ���װ������ ����
��3��Ҫ�ռ�һƿ�ϴ�������������˿ȼ��ʵ�飬���ѡ����ռ�װ������ ������ʼ�ռ�ʱӦע�⣺�� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ָ������ͼʾ���й�������ʵ��װ�û�ʵ������е�һ�����Դ���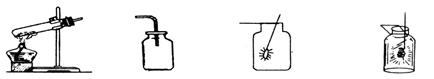
��1������ ��2���ռ� ��3������ ��4����˿ȼ��
��1�� ��2�� -
��3�� ��4�� -
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com