
ĻÖÓŠHClÓėCaCl2µÄ»ģŗĻČÜŅŗ£¬ĪŖĮĖ·ÖĪö»ģŗĻČÜŅŗÖŠHClŗĶCaCl2µÄÖŹĮæ·ÖŹż£¬
Éč¼ĘĮĖČēĻĀŹµŃé·½°ø£ŗ
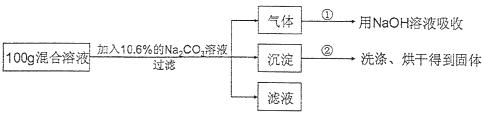
”¾ŹµŃ鏿¾Ż”æŹµŃé¹²¼ĒĀ¼ĮĖĮ½×鏿¾Ż£ŗµŚ¢Ł×飬ĘųĢåĪüŹÕĶźČ«ŗó£¬NaOHČÜŅŗÖŹĮæŌö¼Ó4.4g£»µŚ¢Ś×飬³ĮµķĶźČ«ŗ󣬾¹żĀĖ”¢Ļ“µÓ”¢ŗęøÉŗóµĆµ½¹ĢĢåµÄÖŹĮæĪŖ10g”£
øł¾ŻŹµŃéÉč¼Ę¼°ÓŠ¹ŲŹż¾Ż½ųŠŠ·ÖĪöÓė¼ĘĖć£ŗ
£Ø1£©»ģŗĻČÜŅŗÖŠHClµÄÖŹĮæ·ÖŹżĪŖ ”££ØÖ»Š“½į¹ū£©
£Ø2£©»ģŗĻČÜŅŗÖŠCaCl2µÄÖŹĮæ·ÖŹżĪŖ¶ąÉŁ£æ£ØŠ“³ö¼ĘĖć¹ż³Ģ£©
£Ø3£©ŹµŃéÖŠ»ģŗĻČÜŅŗÓėNa2CO3ČÜŅŗĒ”ŗĆĶźČ«·“Ó¦£¬¾¹żĀĖŗóĖłµĆ”°ĀĖŅŗ”±ÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ ”££Ø²»æ¼ĀĒ¹żĀĖÖŠµÄĖšŹ§”£Ö»Š“½į¹ū£¬±£ĮōŠ”ŹżµćŗóŅ»Ī»£©
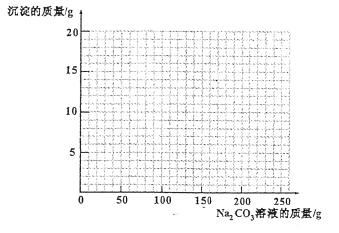
£Ø4£©ČōŌŚ100g»ģŗĻČÜŅŗÖŠ²»¶Ļ¼ÓČė10.6% µÄNa2CO3µÄČÜŅŗ£¬ĒėÄć»³ö¼ÓČėNa2CO3ČÜŅŗµÄÖŹĮæÓė²śÉś³ĮµķµÄÖŹĮæµÄ¹ŲĻµĶ¼”££ØŌŚ“šĢāæصÄ×ų±źĶ¼ÖŠ×÷Ķ¼£©
£Ø1£©7.3%£Ø1·Ö
£Ø2£©£Ø¹²4·Ö£¬ÉčĪ“ÖŖŹż”¢×÷“šŗĶµ„Ī»¹²0.5·Ö£¬»Æѧ·½³ĢŹ½0.5·Ö£¬½ØĮ¢¹ŲĻµŹ½1·Ö£¬x½į¹ū1·Ö£¬ÖŹĮæ·ÖŹż¼ĘĖć1·Ö£©
½ā£ŗÉč»ģŗĻČÜŅŗÖŠCaCl2µÄÖŹĮæĪŖx”£
CaCl2+Na2CO3£½CaCO3”ż+2NaCl
111 100
|
x 10g
x = 11.1g
»ģŗĻČÜŅŗÖŠCaCl2µÄÖŹĮæ·ÖŹż=£Ø11.1g”Ā100g£©”Į100%=11.1%
“š£ŗ»ģŗĻČÜŅŗÖŠCaCl2µÄÖŹĮæ·ÖŹżĪŖ11.1%”£
£Ø3£©8.2%£Ø1·Ö£©
|
£Ø4£©×÷Ķ¼¼ūÓŅĶ¼ĒśĻߣع²1·Ö£©
ĖµĆ÷£ŗµŚ¢ÅĢāĶźČ«ÕżČ·øų1·Ö”£µŚ¢ĘĢā¹²¼Ę4·Ö£ŗÉčĪ“ÖŖŹż”¢×÷“šŗĶµ„Ī»¹²0.5·Ö£¬»Æѧ·½³ĢŹ½0.5·Ö£¬½ØĮ¢¹ŲĻµŹ½1·Ö£¬x½į¹ū1·Ö£¬ÖŹĮæ·ÖŹż¼ĘĖć1·Ö”£Ī“ÖŖŹżÉč¼ĘŗĻĄķ¾łøų·Ö£ØČēÉčĪŖx»ņxg¾łæÉ£©£»Ī“ÖŖŹżÉč¼Ę“ķĪó”¢ĪŽ×÷“š£Ø“š°øµÄŹż¾Ż“ķĪ󣬲»Ó°Ļģøų·Ö£©ŗĶµ„Ī»ŌĖĖć“ķĪóÖŠÓŠ1Ļī¼“æŪ0.5·Ö£»Ö»Š“»Æѧ·“Ó¦·½³ĢŹ½£¬ĒŅÕżČ·øų0.5·Ö£»Ļą¶Ō·Ö×ÓÖŹĮæ¼ĘĖć“ķĪóµÄ£¬Ö»ŌŚ¼ĘĖć½į¹ūÉĻæŪ·Ö£¬²»Ó°Ļģ¹ŲĻµŹ½µÄĘĄ·Ö£»ÓĆ”°¹ŲĻµŹ½”±·ØĄ“ŌĖĖćµÄ£¬Ö»ŅŖŗĻĄķ²¢ÕżČ·µÄ¾łøų·Ö£»Ö»ÓŠ“š°ø£Øƻӊ¼ĘĖć¹ż³Ģ£©µÄĒéæö²»øų·Ö”£µŚ¢ĒĢāĶźČ«ÕżČ·øų1·Ö£¬Ģī8.193%²»æŪ·Ö”£µŚ¢ČĢāׄהʚµć”¢ÕŪµć¼°ĒśĻß×ߏĘĘĄ·Ö£¬“ķŅ»“¦²»øų·Ö£»ĒśĻßĮ½¶Ī¾łĪŖÖ±Ļߣ¬Ę«²īĢ«“ó²»øų·Ö£»ŌŚĘšµć”¢ÕŪµć¼°ĒśĻß×ߏĘƻӊ“ķĪóµÄĒéæöĻĀ£¬»µĆ²»µ½Ī»£ØČēÖ»»µ½Na2CO3ČÜŅŗÖŹĮæĪŖ200g“¦£¬ĒŅÕżČ·£©µÄøų0.5·Ö”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 12”¢³£ĪĀĻĀ£¬ÓŠŠ©ŃĪČēCaCl2”¢NaClµČĪļÖŹµÄĖ®ČÜŅŗ³ŹÖŠŠŌ£»ÓŠŠ©ŃĪČēNa2CO3µČĪļÖŹµÄĖ®ČÜŅŗ³Ź¼īŠŌ£»ÓŠŠ©ŃĪČēNH4ClµČĪļÖŹµÄĖ®ČÜŅŗ³ŹĖįŠŌ£®ĻÖÓŠHClÓėCaCl2µÄ»ģŗĻČÜŅŗ£¬ĻņĘäÖŠÖšµĪ¼ÓČė¹żĮæijĪļÖŹX£¬ČÜŅŗµÄpHĖęµĪČėXµÄĮæµÄ±ä»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ŌņXŹĒ£Ø””””£©
12”¢³£ĪĀĻĀ£¬ÓŠŠ©ŃĪČēCaCl2”¢NaClµČĪļÖŹµÄĖ®ČÜŅŗ³ŹÖŠŠŌ£»ÓŠŠ©ŃĪČēNa2CO3µČĪļÖŹµÄĖ®ČÜŅŗ³Ź¼īŠŌ£»ÓŠŠ©ŃĪČēNH4ClµČĪļÖŹµÄĖ®ČÜŅŗ³ŹĖįŠŌ£®ĻÖÓŠHClÓėCaCl2µÄ»ģŗĻČÜŅŗ£¬ĻņĘäÖŠÖšµĪ¼ÓČė¹żĮæijĪļÖŹX£¬ČÜŅŗµÄpHĖęµĪČėXµÄĮæµÄ±ä»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ŌņXŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 14”¢ŅŃÖŖ³£ĪĀĻĀŠķ¶ąŃĪČēCaCl2”¢NaClµČĪļÖŹµÄĖ®ČÜŅŗ³ŹÖŠŠŌ£®ĻÖÓŠHClÓėCaCl2µÄ»ģŗĻČÜŅŗ£¬ĻņĘäÖŠÖšµĪµĪČė¹żĮæµÄĻĀĮŠĪļÖŹÖŠµÄijĪļÖŹX£¬ČÜŅŗµÄpHĖęµĪČėXµÄĮæµÄ±ä»Æ¹ŲĻµČēĶ¼ĖłŹ¾£¬ŌņXŹĒ£Ø””””£©
14”¢ŅŃÖŖ³£ĪĀĻĀŠķ¶ąŃĪČēCaCl2”¢NaClµČĪļÖŹµÄĖ®ČÜŅŗ³ŹÖŠŠŌ£®ĻÖÓŠHClÓėCaCl2µÄ»ģŗĻČÜŅŗ£¬ĻņĘäÖŠÖšµĪµĪČė¹żĮæµÄĻĀĮŠĪļÖŹÖŠµÄijĪļÖŹX£¬ČÜŅŗµÄpHĖęµĪČėXµÄĮæµÄ±ä»Æ¹ŲĻµČēĶ¼ĖłŹ¾£¬ŌņXŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com