
�밴Ҫ����ա�
(1)��д����Ӧ�Ļ�ѧ���Ż����ƣ�
��2����ԭ��______���ڣ�2�۵�þԪ��________����2Cl��________����H2O2________��
(2)���H��C��O��Na 4��Ԫ����ѡ��ǡ����Ԫ�أ���ɷ�������Ҫ������ʣ���������ѧʽ��д����Ӧ�Ŀո��ϣ�
����ȼ�յĹ�̬�ǽ���������________��
��һ���ж�����̬�������������������______��
������Ԫ����ɵ��Σ�����Һ�ʼ��ԣ���������ϴ�Ӽ�����________��
�ܲ���ֲ��Ĺ�����ã�������ũ����������ϵ���________��
������л������Ȼ������Ҫ�ɷ֣�______��
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| +2 |
| Cu |
| +2 |
| Cu |
| ||
| Ҷ���� |
| ||
| Ҷ���� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1��H �� |
 |
2��He �� | ||||||
| 3��Li � |
4��Be �� |
5��B �� |
6��C ̼ |
7��N �� |
8��O �� |
9��F �� |
10��Ne �� | |
| 12��Mg þ |
13��A1 �� |
14��Si �� |
15��P �� |
16��S �� |
X��C1 �� |
18��Ar � | ||
 ����ʾ�����ӷ�����
����ʾ�����ӷ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
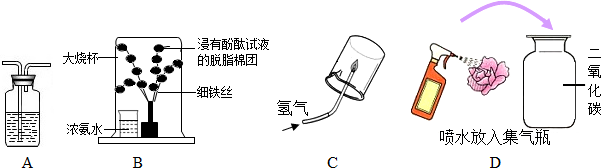
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com