



 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ����������Ƭ�����꼶ѧҵˮƽ���Ի�ѧ�Ծ����������� ���ͣ������
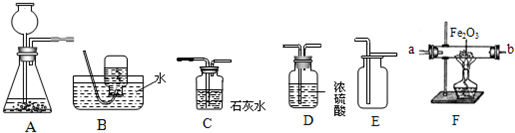
��6�֣���ͼΪʵ�����г����������Ʊ�������ռ��IJ�����������װʵ��װ��ʱ�����ظ�ѡ�����������Ը�����ĿҪ�ش��������⣺
��1������ʵ��������ϡ���������ʯ��ȡ���ռ�����Ķ�����̼���壬��ѡ����������˳��Ϊ �������������ĸ�����йػ�ѧ����ʽΪ
_____________________��
��2����֪ij���������CO2��CO��H2����������ɣ����øû������ⶨ��������Ʒ�Ĵ��ȣ���������������ΪC1��D1��F��D2��C2����������ͨ��װ�ã����з����ķ�Ӧ��ǡ����ȫ���У���
��װ��C1�е������� ��
��װ��D1������ ��
��д��CO��Fe2O3��Ӧ�Ļ�ѧ����ʽ ��
���ڲⶨ��������Ʒ�Ĵ���ʱ����ͬѧ��C2װ�õ����������������������������Ӷ�������������Ʒ�Ĵ��ȣ�����Ϊ�������� ����ƫ����ƫС������ȷ������ȷ����֮һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ���������������п���ѧģ���Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�������������о��꼶Ƭ��ѧҵˮƽ���Ի�ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com