
ij��ѧ��ȤС���ˮ����ͨ�����ȵĽ�̿�õ��Ļ��������Ҫ�ɷֲ�������Ȥ��ͬѧ�Ǿ���ͨ��ʵ�����̽����
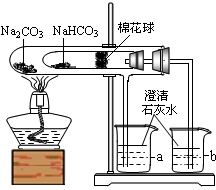
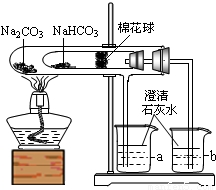
��������롿�û��������Ҫ�ɷ�Ϊһ����̼��������̼��������ˮ������
���������ϡ�a����ˮ����ͭ��ˮ�ɰ�ɫ��Ϊ��ɫ��
b����ʯ���ǹ����������ƺ������ƵĻ���
c��������һ����̼�����ڼ��ȵ�������������ͭ��Ӧ��
��ʵ����̡�ͬѧ������ʦ��ָ�������������ͼ��ʾװ�ã���������ʵ��(���ּг���������ȥ)��
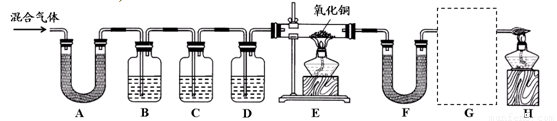
��1��װ��A����ˮ����ͭ������װ��B�г���ʯ��ˮ����ǡ��ɴ˵ó��Ľ���Ϊ ��д��װ��B�з�Ӧ�Ļ�ѧ����ʽ ��
��2��װ��C��D�е�ҩƷ�ֱ�Ϊ ��
��3��ͬѧ��ͨ���۲�װ��E��F�е������֤���˻�����к���������Ϊ��֤������������Ĵ��ڣ�װ��G�е�ҩƷ������ͽ����� ��
��ʵ����ۡ�������ȷ��
��ʵ�鷴˼��
�������ۣ�ͬѧ�ǽ���ͼ��װ��C~H�����˼��Ľ����װ������ͼ��ʾ��
��3��������м�ʯ�ҵ�����Ϊ ��
|
|
ʵ����� |
ʵ������ |
ʵ����� |
|
�� |
�ڼ��촦��ȼ���壬 �� |
�� |
�� |
|
�� |
��
|
�� |
�� |
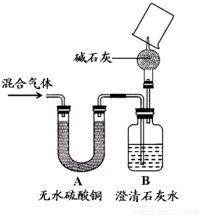
��4��ͬѧ��Ϊ����֤ͨ������ܺ�����ijɷ֣��������е�ʵ��Ϊ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
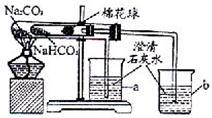 27��ij��ѧ��ȤС���ͬѧ��ѧϰ�˴��Na2CO3�������ʺ����뵽����������ͷʱ���õ�����С�մ�NaHCO3�������Dz������۵�С�մ���Ʒ����������ʵ��̽����
27��ij��ѧ��ȤС���ͬѧ��ѧϰ�˴��Na2CO3�������ʺ����뵽����������ͷʱ���õ�����С�մ�NaHCO3�������Dz������۵�С�մ���Ʒ����������ʵ��̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011������ʡ�������п���ѧ����Ӧ��ϰ�������������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com