
| A����ij�¶��£���35g KCl��Һ���ɵõ�7gKCl���壬��ԭ��Һ������������Ϊ20% |
| B����100g 98%Ũ����ϡ����10%ϡ���ᣬ���ˮ������Ϊ880g |
| C����50g10% NaCl��Һ��Ϊ20%��NaCl��Һ����������25gˮ |
| D����20��ʱ��40g KNO3����ܽ���100gˮ�У���Ȼ��8.4g KNO3���ܣ�������Һ��KNO3����������31.6% |
| 7g |
| 35g |
| 31.6g |
| 100g+31.6g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ƽ��ȡ5.0g���� |
| B��������Ϊ10mL����Ͳ��ȡ�����ˮ |
| C���ܽ�����ʱ��ʹ�õIJ����������ձ��������� |
| D�������ƺõ���Һװ����ƿ�У�����ƿ�������ϱ�ǩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ϡHCl��NaOH��Һ��Ӧ��pH�仯���ߣ���ͼ�����ܵó��Ľ�����ȷ���ǣ�������
��ͼ��ϡHCl��NaOH��Һ��Ӧ��pH�仯���ߣ���ͼ�����ܵó��Ľ�����ȷ���ǣ�������| A���÷�Ӧ��ϡ�������NaOH��Һ |
| B��a����ָ����NaOH��Һ������ |
| C��A��ʱ����Һ������ΪNaOH��NaCl |
| D��B���ʾϡ����ͼ����NaOH��Һ����һ����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
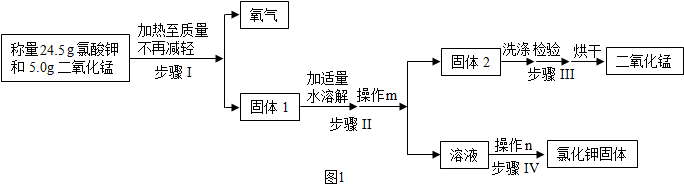

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
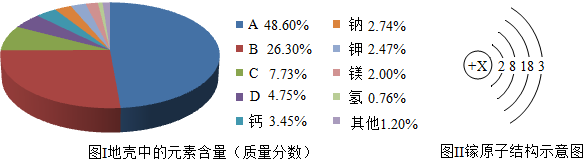
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��һ�����ʱ�ǩ��һ���֣���ش�
��ͼ��һ�����ʱ�ǩ��һ���֣���ش��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com