
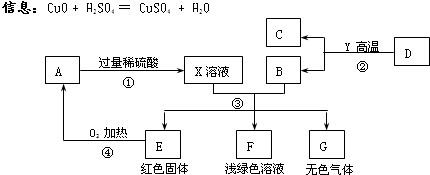
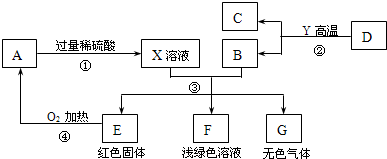
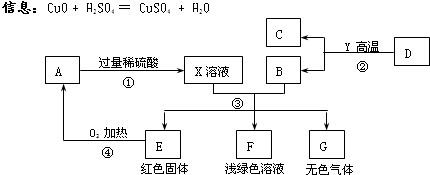
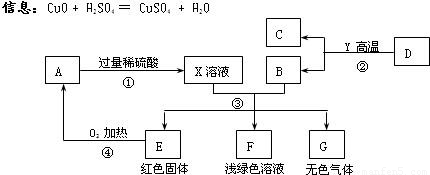
AĪŖŗŚÉ«¹ĢĢ壬A”¢C”¢D”¢Y¶¼ŹĒŃõ»ÆĪļ£¬EŹĒµ„ÖŹ£¬F”¢GĪŖ³£¼ūµÄĪļÖŹ£¬ĘäÖŠB”¢E”¢GŹōÓŚµ„ÖŹ£¬·“Ó¦¢ŚŹĒĮ¶Ģś¹¤ŅµÖŠµÄÖ÷ŅŖ·“Ó¦£¬ĻĀĶ¼ŹĒĖüĆĒÖ®¼äµÄĻą»„×Ŗ»Æ¹ŲĻµ£®Ēė»Ų“š£ŗ
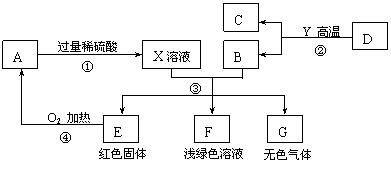
(1)XČÜŅŗÖŠĖłŗ¬ÓŠµÄČÜÖŹĪŖ £¬¹ĢĢåAµÄ»ÆѧŹ½ĪŖ________£¬FČÜŅŗĻŌĒ³ĀĢÉ«£¬ĘäÖŠĖłŗ¬ÓŠµÄČÜÖŹŹĒ__________”£
(2) Š“³ö·“Ó¦¢ŪÖŠÉś³ÉĪŽÉ«ĘųĢåµÄ»Æѧ·½³ĢŹ½ £¬
(3) Öø³öĪļÖŹGŗĶAŌŚ¼ÓČČĢõ¼žĻĀ·¢ÉśµÄ»Æѧ·“Ó¦µÄ»ł±¾·“Ó¦ĄąŠĶ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2009-2010ѧğ½ĖÕŹ”Õņ½ŹŠÕņ½ĖÄÖŠ¾ÅÄź¼¶£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com