
| A”¢¢Ś¢Ü¢Ż | B”¢¢Ś¢Ū¢Ü¢Ż |
| C”¢¢Ś¢Ū¢Ż | D”¢¢Ł¢Ś¢Ū¢Ü¢Ż |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| +2 |
| MgO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ļÓ“óĆ×£¬Ņ»°ćÖøļÓŗ¬Į泬±źµÄ“óĆ×£¬ļÓĶس£Ķعż·ĻĖ®ÅÅČė»·¾³ÖŠ£¬ŅżĘšÖ²ĪļµÄĪüŹÕ£¬ČēĶ¼ĪŖŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¹ŲŠÅĻ¢£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ļÓ“óĆ×£¬Ņ»°ćÖøļÓŗ¬Į泬±źµÄ“óĆ×£¬ļÓĶس£Ķعż·ĻĖ®ÅÅČė»·¾³ÖŠ£¬ŅżĘšÖ²ĪļµÄĪüŹÕ£¬ČēĶ¼ĪŖŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¹ŲŠÅĻ¢£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A”¢ļÓŌŖĖŲµÄŌ×ÓŠņŹżĪŖ48 |
| B”¢ļÓŌŖĖŲµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ112.4g |
| C”¢ļÓŌŖĖŲµÄÖŠ×ÓŹżĪŖ48 |
D”¢ļÓŌŖĖŲµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ¹ž¶ū±õĪ÷æĶÕ¾ŹĒĪŅ¹śøßŗ®µŲĒų×ī“óµÄæĶŌĖ×ŪŗĻ½»ĶØŹąÅ¦£¬³µÕ¾·ÖÉĻĻĀĪå²ć£¬ÓÉl8×łÕ¾ĢØ”¢22ĢõĢśĀ·µ½·¢Ļß”¢øß¼Üŗņ³µŹŅ£¬ŅŌ¼°¹«½»ŹąÅ¦Õ¾”¢µŲĢś³µÕ¾”¢³ö×ā³µÕ¾µČ×é³É£®ĻĀĮŠŠšŹö“ķĪóµÄŹĒ£Ø””””£©
¹ž¶ū±õĪ÷æĶÕ¾ŹĒĪŅ¹śøßŗ®µŲĒų×ī“óµÄæĶŌĖ×ŪŗĻ½»ĶØŹąÅ¦£¬³µÕ¾·ÖÉĻĻĀĪå²ć£¬ÓÉl8×łÕ¾ĢØ”¢22ĢõĢśĀ·µ½·¢Ļß”¢øß¼Üŗņ³µŹŅ£¬ŅŌ¼°¹«½»ŹąÅ¦Õ¾”¢µŲĢś³µÕ¾”¢³ö×ā³µÕ¾µČ×é³É£®ĻĀĮŠŠšŹö“ķĪóµÄŹĒ£Ø””””£©| A”¢Ī÷æĶÕ¾µÄ½Ø³É£¬»ŗ½āĮĖ¹ž¶ū±õ»š³µÕ¾µÄŃ¹Į¦ |
| B”¢Ī÷æĶÕ¾½ØÉ菱ÓĆN-Y”°“óĮæµÄøֲģ¬øÖ²ÄŹōÓŚø“ŗĻ²ÄĮĻ |
| C”¢ÄæĒ°£¬Į¬½ÓĪ÷æĶÕ¾µÄŹŠÄŚ¹«½»ĻµĶ³Ņ²ČÕŅęĶźÉĘ |
| D”¢Ī÷æĶÕ¾æÉŅŌĀś×ćĀĆæĶĪŽ·ģĻ¶×Ŗ³Ė³µ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| AӢ4.6% | BӢ9.2% |
| CӢ5.3% | DӢ11.2% |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢ÖĪĮĘĪøĖį¹ż¶ąÖ¢ 2HCl+Ca£ØOH£©2=CaCl2+2H2O ÖŠŗĶ·“Ó¦ |
| B”¢ĢśÓėĻ”ĮņĖį·“Ó¦ÖĘĀČĘų 2Fe+3H2SO4=Fe2£ØSO4£©3+3H2”ü ÖĆ»»·“Ó¦ |
| C”¢ÓźĖ®³ŹĖįŠŌµÄŌŅņ CO2+H2O=H2CO3 »ÆŗĻ·“Ó¦ |
| D”¢ĪüŹÕ¶žŃõ»ÆĘųĢå CO2+2NaOH=Na2CO3+H2O ø“·Ö½ā·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢Ņ×ČÜÓŚĖ®£¬ČܽāŹ±·Å³ö“óĮæµÄČČ |
| B”¢ÄÜČ„³żÓĶĪŪ£¬æÉ×ö³ų·æµÄĒå½ą¼Į |
| C”¢Ė®ČÜŅŗÄÜŹ¹ŹÆČļČÜŅŗ±äŗģ |
| D”¢¶Ōʤ·ōÓŠĒæĮŅµÄøÆŹ“×÷ÓĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
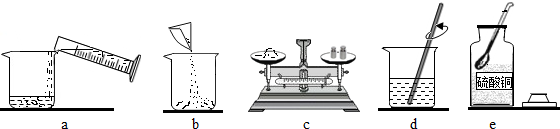
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
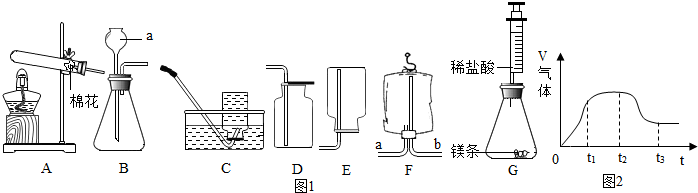
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com