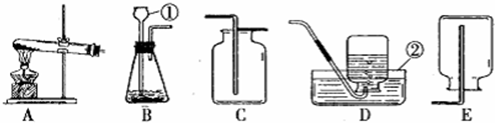
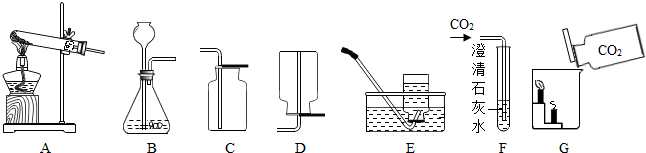
��2012?���ݣ��������ͼʾʵ��װ�ã��ش��й����⣺
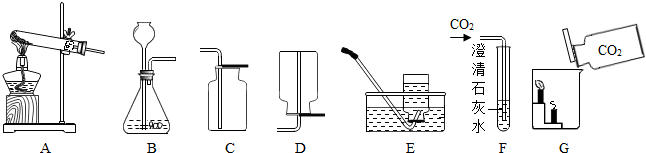
��1��ʵ�����ø��������ȡ����ʱ��Ӧѡ�õķ���װ����
A
A
������ĸ��ͬ������Ӧ�Ļ�ѧ����ʽΪ
���Թܿڷ�һ������������
��ֹ����ʱ������ط�ĩ���뵼����
��ֹ����ʱ������ط�ĩ���뵼����
��
��2��ʵ������ȡ������̼ʱ��Ӧѡ��ķ������ռ�װ����
B��C
B��C
��
��3��F�й۲쵽��ʵ��������
�����ʯ��ˮ����ǣ����а�ɫ�������ɣ�
�����ʯ��ˮ����ǣ����а�ɫ�������ɣ�
��
��Ӧ�Ļ�ѧ����ʽΪ
Ca��OH��2+CO2=CaCO3��+H2O
Ca��OH��2+CO2=CaCO3��+H2O
��
��4��Gʵ����˵��������̼���е�������
����ȼ�գ�Ҳ��֧��ȼ�գ����ܶȱȿ�����
����ȼ�գ�Ҳ��֧��ȼ�գ����ܶȱȿ�����
����CO
2������
���
���
��
��5�������£����⣨H2S����һ���г�������ζ�����壺ʵ���ҿ�����������FeS�������ϡ���ᷴӦ�Ƶã��÷�Ӧ�Ļ�ѧ����ʽΪFeS+H
2S0
4=H
2S��+FeSO
4��ʵ������ȡ��������Ӧѡ�õķ���װ����
B
B
��������
��Ӧ��״̬�ǹ����Һ���ҷ�Ӧ����Ҫ����
��Ӧ��״̬�ǹ����Һ���ҷ�Ӧ����Ҫ����
��




 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�