ij����С��������þ�Ͻ�����о����ⶨ����þ������������
���������ϡ�
��1��������þ������������������ˮ�����ܼ����Ⱥ��ֽܷ�����ˮ����Ӧ�Ľ��������
��2��þ��������þ������������������Һ��Ӧ����������������������������������Һ�������·�Ӧ��2Al+2NaOH+2H
2O=2NaAlO
2+3H
2�� Al��OH��
3+NaOH=NaAlO
2+2H
2O
��������ơ���������ʵ�����ṩ�����ᡢ����������Һ��������ֲ�ͬ��ʵ�鷽����
����һ����þ�Ͻ�
�ⶨ������������
����������þ�Ͻ�
�ⶨ������������
����������þ�Ͻ�
��Һ
�������ղ���
���������ۡ�
1�����������Ƿ�����У����в����е���˵�����ɣ�
��
2���������С���Ա����Ը����ú��ַ�����
�������һ��������������������������������
��
��ʵ����ơ�ʵ��С����ݷ��������������ͼ��ʾ��ͼ�е�����̨��ʡ�ԣ�������ʵ��װ�ã�
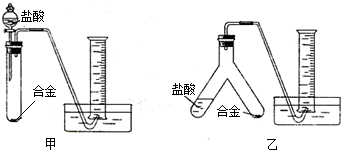
����Ϊѡ��
ѡ����ң�װ�ý���ʵ�������������С��
���������ۡ�
��1��С���Աʵ���ͨ�����������ձ���Ϊ��������ʵ�鷽�������ڲ��������������ײ��������������⣮���ǰ���������ʽ��������˷��������������������·�������ƣ��ڡ��Ϸ���д��Ӧ���Լ��Ͳ�������
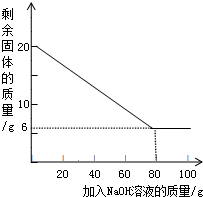
2��С���Ա�����µIJⶨ������ȡ20g��þ�Ͻ��гɽ�����ĩ��100g����������Һƽ���ֳ�5�����μ��룬��ַ�Ӧ���˳����壬����ϴ�ӡ����������ʵ������еõ��IJ���������ͼ�����£�
| ��NaOH��Һ�Ĵ��� |
��һ�� |
�ڶ��� |
������ |
�� |
| ʣ����������/g |
1 6.5 |
n |
9.5 |
�� |
��1��С���Ա����þ�Ͻ��гɽ�����ĩ��Ŀ����
��
��2�������������ݿ�֪���ϱ���n��ֵΪ
��
��3���ý�����ĩ��Al����������Ϊ
%
��4����ʽ���㣺��������������Һ��������������Ϊ���٣�������̣�
��

 ij����С��������þ�Ͻ�����о����ⶨ����þ������������
ij����С��������þ�Ͻ�����о����ⶨ����þ������������



 ij����С��������þ�Ͻ�����о����ⶨ����þ������������
ij����С��������þ�Ͻ�����о����ⶨ����þ������������ �ⶨ����������������
�ⶨ����������������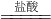 �ⶨ������������
�ⶨ������������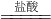 ��Һ
��Һ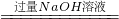 �������ղ���
�������ղ���
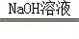 �ⶨ������������
�ⶨ������������ �ⶨ������������
�ⶨ������������ ��Һ
��Һ �������ղ���
�������ղ���

 �ⶨ������������
�ⶨ������������  �ⶨ������������
�ⶨ������������ ��Һ
��Һ �������ղ���
�������ղ���
