
С��ͬѧ�������ʵ��װ�ã�����̨������ʡ�ԣ��Ʊ�CO2����֤CO2����NaOH��Ӧ��
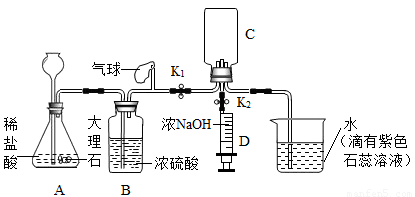
��1��װ��B��������______
��2���벹�仭��װ��C��a��b������
��3��С�����ʵ����ִ�װ�������Բ��㣬����Ӧ����һ������ƿF������ΪӦ����_____(��װ�ñ��)֮�����װ��______(���Լ����)ϴ��ƿF��
A��ŨNaOH��Һ B������ʯ��ˮ C�� ����NaHCO3��Һ D������Na2CO3��Һ
��4����ֹˮ��K1����������ԣ�����ҩƷ��ʼʵ�飬���۲���װ��E��������__________________________����ȷ��װ��C�ռ���CO2
��5����װ��C���ռ���CO2���ر�ֹˮ��K2����ע����D�е�5mLŨ����������ѹ�뵽װ��C�У���ѧ����ʽΪ_______________________________________,�۲쵽��������____________________________________.
(6)Сƽͬѧ��Ϊ֤��CO2�� NaOH��Ӧ����Ӧ��ע������D��ŨNaOH��Һ����________,����һ��ʵ�飬��Ŀ����__________________________��
��1�����������̼������� ���ն�����̼�е�ˮ�֣�
��2��
��3��AB ����̼��������Һ
��4����Һ��Ϊ��ɫ
��5��2NaOH + CO2 = Na2CO3 + H2O ��E�е����ڵ�Һ������ձ��е�Һ��
��6��ϡ���ᣨ����� ϡ���ᣩ ��֤������̼����
����������1��Ũ���������ˮ�ԣ������ն�����̼�е�ˮ�֣���2��������̼���ܶȱȿ����Ĵ�a����ֻ�������뼯��ƿ�У�b����Ҫ���뼯��ƿ�ײ������ܱ�֤����ƿ���ռ����㹻�Ķ�����̼����3��̼��������Һ�������ᷴӦ����ȥ�ӷ��������Ȼ������壬ͬʱ������̼����̼�����Ʒ�Ӧ ��4��������̼��ˮ��Ӧ������̼�ᣬ̼����Һ�����ԣ���ʹ��ɫʯ����Һ���ɫ����5�����������������̼��Ӧ������̼���ƺ�ˮ�������˶�����̼������ƿ�е�ѹǿ��С���ڴ���ѹǿ�������£�������Һ��ѹ�뵼���У�ʹ�����ڵ�Һ������ձ��е�Һ�棻��6��������̼���������Ʒ�Ӧû���������ʼ���ϡ��������ɵ�̼��������ϡ���ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼���������ݡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ��ɽ�и��е�ѧУ�������Ի�ѧ ���ͣ�059
С��ͬѧ�������ʵ��װ��(����̨������ʡ��)�Ʊ�CO2����֤CO2����NaOH��Ӧ��
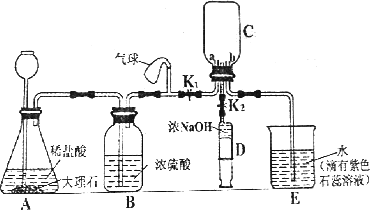
(1)װ��B��������________��
(2)�벹�仭��װ��C��a��b������
(3)С�����ʵ����ִ�װ�������Բ��㣬����Ӧ����һ������ƿF������ΪӦ����________(��װ�ñ��)֮�����װ��________��(���Լ����)ϴ��ƿF��
a��ŨNaOH��Һ
b������ʯ��ˮ
c������NaHCO3��Һ
d������Na2CO3��Һ
(4)��ֹˮ��K1����������ԣ�����ҩƷ��ʼʵ�飬���۲���װ��E��������________����ȷ��װ��C�ռ���CO2��
(5)��װ��C���ռ���CO2���ر�ֹˮ��K2����ע����D�е�5 mLŨ����������ѹ�뵽װ��C�У���ѧ����ʽΪ________���۲쵽��������________��
(6)Сƽͬѧ��Ϊ֤��CO2��NaOH��Ӧ����Ӧ��ע������D��ŨNaOH��Һ����________������һ��ʵ�飬��Ŀ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С��ͬѧ�������ʵ��װ�ã�����̨������ʡ�ԣ��Ʊ�CO2����֤CO2����NaOH��Ӧ��
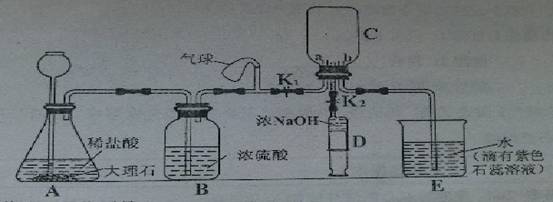 ��1��װ��B��������______
��1��װ��B��������______
��2���벹�仭��װ��C��a��b������
��3��С�����ʵ����ִ�װ�������Բ��㣬����Ӧ����һ������ƿF������ΪӦ����_____(��װ�ñ��)֮�����װ��______(���Լ����)ϴ��ƿF��
a��ŨNaOH��Һ b������ʯ��ˮ c�� ����NaHCO3��Һ d������Na2CO3��Һ
��4����ֹˮ��K1����������ԣ�����ҩƷ��ʼʵ�飬���۲���װ��E��������__________________________����ȷ��װ��C�ռ���CO2
��5����װ��C���ռ���CO2���ر�ֹˮ��K2����ע����D�е�5mLŨ����������ѹ�뵽װ��C�У���ѧ����ʽΪ________________________________________,�۲쵽��������________________________________________________________.
(6)Сƽͬѧ��Ϊ֤��CO2�� NaOH��Ӧ����Ӧ��ע������D��ŨNaOH��Һ����________,����һ��ʵ�飬��Ŀ����__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С��ͬѧ�������ʵ��װ�ã�����̨������ʡ�ԣ��Ʊ�CO2����֤CO2����NaOH��Ӧ��
��1��װ��B��������______
��2���벹�仭��װ��C��a��b������
��3��С�����ʵ����ִ�װ�������Բ��㣬����Ӧ����һ������ƿF������ΪӦ����_____(��װ�ñ��)֮�����װ��______(���Լ����)ϴ��ƿF��
a��ŨNaOH��Һ b������ʯ��ˮ c�� ����NaHCO3��Һ d������Na2CO3��Һ
��4����ֹˮ��K1����������ԣ�����ҩƷ��ʼʵ�飬���۲���װ��E��������__________________________����ȷ��װ��C�ռ���CO2
��5����װ��C���ռ���CO2���ر�ֹˮ��K2����ע����D�е�5mLŨ����������ѹ�뵽װ��C�У���ѧ����ʽΪ________________________________________,�۲쵽��������________________________________________________________.
(6)Сƽͬѧ��Ϊ֤��CO2�� NaOH��Ӧ����Ӧ��ע������D��ŨNaOH��Һ����________,����һ��ʵ�飬��Ŀ����__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С��ͬѧ�������ʵ��װ�ã�����̨������ʡ�ԣ��Ʊ�CO2����֤CO2����NaOH��Ӧ��

��1��װ��B��������______
��2���벹�仭��װ��C��a��b������
��3��С�����ʵ����ִ�װ�������Բ��㣬����Ӧ����һ������ƿF������ΪӦ����_____(��װ�ñ��)֮�����װ��______(���Լ����)ϴ��ƿF��
| A��ŨNaOH��Һ | B������ʯ��ˮ | C������NaHCO3��Һ | D������Na2CO3��Һ |
��4����ֹˮ��K1����������ԣ�����ҩƷ��ʼʵ�飬���۲���װ��E��������__________________________����ȷ��װ��C�ռ���CO2
��5����װ��C���ռ���CO2���ر�ֹˮ��K2����ע����D�е�5mLŨ����������ѹ�뵽װ��C�У���ѧ����ʽΪ_______________________________________,�۲쵽��������____________________________________.
(6)Сƽͬѧ��Ϊ֤��CO2�� NaOH��Ӧ����Ӧ��ע������D��ŨNaOH��Һ����________,����һ��ʵ�飬��Ŀ����__________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com