
����ȡ֮�����Ļ���ԭ�ϱ��⣬�Ӻ�ˮ�п���ȡ�����ԭ�ϣ���ͼ�ǹ�ҵ�Ժ�ˮ���м����ۺ����õ�ʾ��ͼ������ͼʾ��գ�
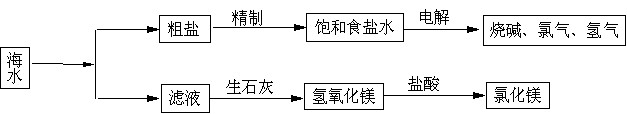
(1)����Һ(���Ȼ�þ)�м�����ʯ����ȡ������þʱ��������Ӧ�Ļ�ѧ����ʽ��
��
(2)ij���⻯ѧ�С��ȡ��ˮ���������ᾧ��Ȼ����ˣ��õ����Σ�����ʱ�������õ��IJ��������� ��
(3)���������д���CaCl2�����ʣ�Ϊ�˳�ȥCaCl2�����ڡ����Ʊ���ʳ��ˮ��ʱ���������
(д��ѧʽ)��Һ���ٹ��ˣ������������� (���Լ�����)���ɣ������ˮ��Ϫˮ�к��е�Ca2����Mg2���϶࣬����ˮ���� ���ճ������н���ˮ��Ca2����Mg2���ķ����� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ��ĩ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com