
ŹµŃéŹŅ³£ÓĆČēĶ¼ĖłŹ¾×°ÖĆĄ“ŃŠ¾æĘųĢåµÄÖĘČ”ŗĶŠŌÖŹ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ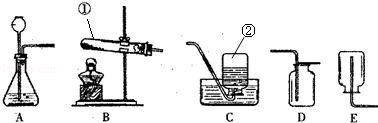
£Ø1£©Š“³öŅĒĘ÷µÄĆū³Ę£ŗ¢Ł ¢Ś ”£A×°ÖĆÖŠ³¤¾±Ā©¶·ĻĀ¶Ė¹ÜæŚÉģČėŅŗĆęĻĀµÄÄæµÄŹĒ ”£
£Ø2£©ÓĆøßĆĢĖį¼ŲÖĘČ”ŃõĘųµÄ»Æѧ·½³ĢŹ½ĪŖ £¬ĄūÓĆøĆ·“Ó¦ÖĘČ”ŃõĘų£¬æÉŃ”ÓƵķ¢Éś×°ÖĆŹĒ £ØĢī×ÖÄø£¬ĻĀĶ¬£©£¬ČōŃ”ÓĆC×°ÖĆŹÕ¼ÆŃõĘų£¬ŹµŃé½įŹųŗóµÄ²Ł×÷Ė³ŠņŹĒĻČ½« £¬Č»ŗó £¬ŅŌ·ĄÖ¹Ė®µ¹Īü½ųČėČȵďŌ¹ÜÖŠ£¬Ōģ³ÉŹŌ¹ÜµÄĘĘĮŃ”£
£Ø3£©ŌŚ¼ŗѧ¹żµÄ³õÖŠ»ÆѧÖŖŹ¶Ąļ£¬ĄūÓĆA×°ÖĆæÉŅŌÖĘČ”¶žŃõ»ÆĢ¼£¬ŹµŃéŹŅ³£ÓĆŹÆ»ŅŹÆÓėĻ”ŃĪĖįÖĘČ”øĆĘųĢ壬»Æѧ·½³ĢŹ½ĪŖ £¬»ł±¾·“Ó¦ĄąŠĶŹĒ £¬ŹÕ¼Æ¶žŃõ»ÆĢ¼Ó¦Ń”ÓĆ ×°ÖĆ”£
£Ø4£©NOĘųĢåÄŃČÜÓŚĖ®£¬Ņ×±»æÕĘųŃõ»Æ£¬ŹÕ¼ÆNOĘųĢåÄÜŃ”ÓƵÄ×°ÖĆŹĒ ”£
£Ø1£©¢ŁŹŌ¹Ü£»¢Ś¼ÆĘųĘ棻·ĄÖ¹ĘųĢå“Ó³¤¾±Ā©¶·ŅŻ³ö£»
£Ø2£©2KMnO4 K2MnO4+MnO2+O2”ü£» B£»µ¼¹ÜŅĘ³öĖ®Ćę£» ĻØĆš¾Ę¾«µĘ£»
K2MnO4+MnO2+O2”ü£» B£»µ¼¹ÜŅĘ³öĖ®Ćę£» ĻØĆš¾Ę¾«µĘ£»
£Ø3£©CaCO3+2HCl£½CaCl2+CO2”ü+H2O£»ø“·Ö½ā·“Ó¦£» D£» £Ø4£©C
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©±źŗÅŅĒĘ÷·Ö±šŹĒŹŌ¹ÜŗĶ¼ÆĘųĘ棻A×°ÖĆÖŠ³¤¾±Ā©¶·ĻĀ¶Ė¹ÜæŚÉģČėŅŗĆęĻĀµÄÄæµÄŹĒ£ŗ·ĄÖ¹ĘųĢå“Ó³¤¾±Ā©¶·ŅŻ³ö£»
£Ø2£©¼ÓČČøßĆĢĖį¼ŲÉś³ÉĆĢĖį¼Ų”¢¶žŃõ»ÆĆĢŗĶŃõĘų£¬·½³ĢŹ½ŹĒ£ŗ2KMnO4 K2MnO4+MnO2+O2”ü£¬øĆ·“Ó¦ŹōÓŚ¹ĢĢå¼ÓČČŠĶ£¬¹ŹŃ”·¢Éś×°ÖĆB”£ČōÓĆCŹÕ¼ÆŃõĘų£¬ŹµŃé½įŹųŗóÓ¦ĻČŅʵ¼¹ÜŗóĻØµĘ£¬·ĄÖ¹Ė®µ¹Īü½ųČėČȵďŌ¹ÜÖŠ£¬Ōģ³ÉŹŌ¹ÜµÄĘĘĮŃ£»
K2MnO4+MnO2+O2”ü£¬øĆ·“Ó¦ŹōÓŚ¹ĢĢå¼ÓČČŠĶ£¬¹ŹŃ”·¢Éś×°ÖĆB”£ČōÓĆCŹÕ¼ÆŃõĘų£¬ŹµŃé½įŹųŗóÓ¦ĻČŅʵ¼¹ÜŗóĻØµĘ£¬·ĄÖ¹Ė®µ¹Īü½ųČėČȵďŌ¹ÜÖŠ£¬Ōģ³ÉŹŌ¹ÜµÄĘĘĮŃ£»
£Ø3£©ŹÆ»ŅŹÆŗĶĻ”ŃĪĖį·“Ӧɜ³ÉĀČ»ÆøĘ”¢Ė®ŗĶ¶žŃõ»ÆĢ¼£¬·½³ĢŹ½ŹĒCaCO3+2HCl£½CaCl2+CO2”ü+H2O£¬øĆ·“Ó¦ŹĒÓÉĢ¼ĖįøĘŗĶŃĪĖįĮ½ÖÖ»ÆŗĻĪļĻą»„½»»»³É·ÖÉś³ÉĢ¼ĖįŗĶĀČ»ÆøĘĮ½ÖÖ»ÆŗĻĪļ£¬Ģ¼Ėį²»ĪČ¶Ø·Ö½āÉś³ÉĖ®ŗĶ¶žŃõ»ÆĢ¼£¬¹ŹŹōÓŚø“·Ö½ā·“Ó¦£¬¶žŃõ»ÆĢ¼µÄĆܶȱČæÕĘų“óĒŅÄÜČÜÓŚĖ®£¬¹ŹŃ”ĻņÉĻÅÅæÕĘų·½·Ø£»
£Ø4£©NOĘųĢåÄŃČÜÓŚĖ®£¬Ņ×±»æÕĘųŃõ»Æ£¬ĖłŅŌ²»ÄÜÓĆÅÅæÕĘų·ØŹÕ¼Æ£¬æÉÓĆÅÅĖ®·ØŹÕ¼Æ”£
æ¼µć£ŗ漲鳣ÓĆĘųĢåµÄ·¢Éś×°ÖĆŗĶŹÕ¼Æ×°ÖĆÓėєȔ·½·Ø



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Öø³öĻĀĮŠĶ¼Ź¾ÖŠÓŠ¹ŲŃõĘųµÄŹµŃé×°ÖĆ»ņŹµŃé²Ł×÷ÖŠµÄŅ»“¦Ć÷ĻŌ“ķĪó”£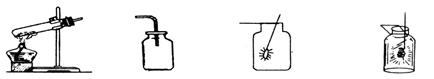
£Ø1£©·¢Éś £Ø2£©ŹÕ¼Æ £Ø3£©ŃéĀś £Ø4£©ĢśĖæČ¼ÉÕ
£Ø1£© £Ø2£© -
£Ø3£© £Ø4£© -
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øł¾ŻĻĀĮŠŹµŃé×°ÖĆĶ¼£¬»Ų“šĪŹĢā”£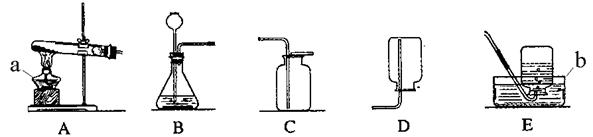
£Ø1£©Š“³ö×°ÖĆÖŠ±źŗÅŅĒĘ÷µÄĆū³Ę£ŗa £¬b ”£
£Ø2£©ČōŃ”ÓĆA×°ÖĆ×÷ĪŖŃõĘųµÄ·¢Éś×°ÖĆ£¬»Æѧ·½³ĢŹ½ŹĒ £¬ŹŌ¹ÜæŚ·ÅŅ»ĶÅĆŽ»ØµÄ×÷ÓĆŹĒ ”£
£Ø3£©ČōŃ”ÓĆB×°ÖĆ×÷ĪŖŃõĘųµÄ·¢Éś×°ÖĆ£¬»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø4£©æÉŃ”ÓĆD×°ÖĆ»ņE×°ÖĆŹÕ¼ÆĒāĘų£¬ŌŅņ·Ö±šŹĒ ”¢ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻÖÓŠĻĀĮŠŅĒĘ÷£ŗ
£Ø1£©ĪüČ”ŗĶµĪ¼ÓÉŁĮæŅŗĢåŹ±ÓĆ_________£¬ÓĆÓŚĻ“ŹŌ¹ÜµÄŅĒĘ÷ŹĒ £ØĢīŅĒĘ÷Ćū³Ę£©
£Ø2£©¼ŅĶ„Š”ŹµŃéĶس£ĄūÓĆÉś»īÖŠ³£¼ūµÄĪļĘ·×öŅ»Š©ŹµŃéŅĒĘ÷µÄĢꓜʷ£¬ÄćČĻĪŖŅ½ÓĆ×¢ÉäĘ÷æÉŅŌ“śĢęÉĻŹöŅĒĘ÷ÖŠµÄ ”¢ ”£
£Ø3£©Č”ÓĆŅ»¶ØĮæŅŗĢåŹŌ¼Į£¬ŠčŹ¹ÓƵÄŅĒĘ÷ÓŠ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ŹĒijæĪĶā»ī¶ÆŠ”×éµÄĶ¬Ń§Éč¼ĘµÄŹµŃéŹŅÖĘČ”CO2²¢¼ģŃéĘäŠŌÖŹµÄ×°ÖĆŹ¾ŅāĶ¼£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ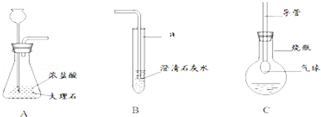
£Ø1£©“śŗÅaĖłÖøŅĒĘ÷µÄĆū³ĘŹĒ £¬A×°ÖĆÖŠ·¢ÉśµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ £»
£Ø2£©½«AÖŠ²śÉśµÄĘųĢåĶØČėµ½BÖŠŅ»»į¶ł£¬Ī“¼ūĘäÖŠ±ä»ė×Ē£®ĄīĻéĶ¬Ń§ČĻĪŖÕāŹĒÅØŃĪĖį»Ó·¢³öµÄHClĘųĢåøÉČÅĖłÖĀ£®ÄćµÄ½āŹĶŹĒ£ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©£ŗ £»
£Ø3£©ÕÅ»ŖĶ¬Ń§ÓĆÉÕĘæŹÕ¼ÆAÖŠ²śÉśµÄĘųĢåŗó£¬ĻņĘäÖŠ¼ÓČė¹żĮæĪŽÉ«ĶøĆ÷µÄMČÜŅŗ×é³ÉC×°ÖĆ£¬Õńµ“£¬·¢ĻÖÉÕĘæÖŠµÄĘųĒņÖš½„±ä“ó£¬ĒŅČÜŅŗŹ¼ÖÕĪŽÉ«ĶøĆ÷£¬ŌņMæÉÄÜŹĒ £ØĢīŠ“Ņ»ÖÖĪļÖŹµÄ»ÆѧŹ½£©£»
£Ø4£©½«AÖŠŅ©Ę·»»³É¹żŃõ»ÆĒāČÜŅŗŗĶ¶žŃõ»ÆĆĢ£®Ōņ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¼×”¢ŅŅ”¢±ūČżĪ»Ķ¬Ń§ŌŚ»ī¶ÆæĪÉĻĢ½¾æĘųĢåµÄÖʱø£®ĄūÓĆĻĀĶ¼ĖłŹ¾×°ÖĆÖĘČ”³£¼ūµÄĘųĢ壬²¢¶ŌĖüĆĒµÄÓŠ¹ŲŠŌÖŹ½ųŠŠŃŠ¾æ£¬Ēėøł¾ŻĢāŅā»Ų“šĻĀĮŠĪŹĢā£®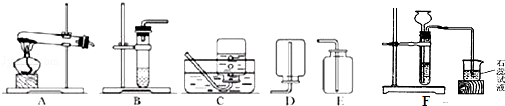
£Ø1£©¼×Ķ¬Ń§ÓĆKMnO4¹ĢĢåÖĘČ”O2£¬·¢Éś×°ÖĆӦєÓĆÉĻĶ¼ÖŠµÄ ×°ÖĆ£ØĢī±ąŗÅ£©£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»µ±O2ŹÕ¼ÆĀś²¢Č”³ö¼ÆĘųĘæŗó£¬Ķ£Ö¹øĆŹµŃéµÄÕżČ·²Ł×÷·½·ØŹĒ
£Ø2£©ŅŅĶ¬Ń§ÓĆÅØŃĪĖįÓė“óĄķŹÆŌŚF×°ÖĆÖŠ·“Ó¦£¬ÖĘČ”CO2²¢¼ģŃéĘäÓŠ¹ŲŠŌÖŹ£¬¹Ū²ģµ½ÉÕ±ÖŠ×ĻÉ«ŹÆČļŹŌŅŗ±äŗģ£¬¶ŌÕāŅ»ĻÖĻóµÄ½āŹĶ²»ŗĻĄķŹĒ £ØĢīŠņŗÅ£©£®
a£®²śÉśµÄCO2Ö±½ÓŹ¹ŹÆČļ±äŗģ
b£®²śÉśµÄCO2ÓėH2O·“Ӧɜ³ÉH2CO3£¬Ź¹ŹÆČļŹŌŅŗ±äŗģ
c£®»Ó·¢³öµÄĀČ»ÆĒāČÜÓŚĖ®£¬Ź¹ŹÆČļŹŌŅŗ±äŗģ
£Ø3£©±ūĶ¬Ń§ŌŚŹµŃéŹŅĀČ»Æļ§¹ĢĢåÓė¼īŹÆ»Ņ¹ĢĢå¹²ČČĄ“ÖĘČ”°±Ęų£ØNH3£©£®³£ĪĀĻĀNH3ŹĒŅ»ÖÖĪŽÉ«”¢ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬ĆܶȱČæÕĘųŠ”£¬¼«Ņ×ČÜÓŚĖ®”£
¢ŁÖĘČ”°±Ęų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ2NH4Cl+Ca£ØOH£©2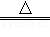 CaCl2+2NH3”ü+2X£®XµÄ»ÆѧŹ½ĪŖ£ŗ £®
CaCl2+2NH3”ü+2X£®XµÄ»ÆѧŹ½ĪŖ£ŗ £®
¢ŚÖĘČ”²¢ŹÕ¼ÆNH3£¬Ó¦øĆ“ÓÉĻĶ¼ÖŠŃ”ŌńµÄŹÕ¼Æ×°ÖĆŹĒ £®
¢ŪNH3ŹĒŅ»ÖÖ¼īŠŌĘųĢ壬øÉŌļŹ±²»ÄÜŃ”ÓĆĻĀĮŠøÉŌļ¼ĮÖŠµÄ £®£ØĢīŠņŗÅ£©£®
A£®¹ĢĢåĒāŃõ»ÆÄĘ B£®ÅØĮņĖį C£®ÉśŹÆ»Ņ£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻÖÓŠĻĀĮŠŅĒĘ÷»ņ×°ÖĆ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ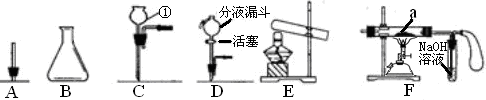
£Ø1£©ŅĒĘ÷¢ŁµÄĆū³ĘŹĒ_____________”£
£Ø2£©ÓĆÉĻĶ¼ŅĒĘ÷×é×°³ÉĘųĢå·¢Éś×°ÖĆ£ŗÓĆKClO3ŗĶMnO2ÖĘO2ӦєµÄ×°ÖĆŹĒ____(Ģī×ÖÄø)£»·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ £»ÓĆ·ĻĢśŠ¼ÓėĻ”ŃĪĖį·“Ó¦ÖĘČ”H2£¬²¢æŲÖĘ²śÉśH2µÄĖŁĀŹ£¬Ó¦Ń”_____£ØĢī×ÖÄø£©”£
£Ø3£©ČōÓĆČēĶ¼×°ÖĆ½ųŠŠ”°ÅÅæÕĘų·Ø”±ŹÕ¼ÆÖĘČ”µÄO2£¬ŃõĘųÓ¦“Ó________(Ģī”°b”±»ņ”°c”±)¶Ėµ¼Čė”£ČōĘæ֊װĀśĖ®£¬ÓĆÅÅĖ®·ØŹÕ¼ÆŃõĘų£¬ŃõĘųÓ¦“Ó______(Ģī”°b”±»ņ”°c”±)¶Ėµ¼Čė”£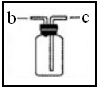
£Ø4£©ČōÓĆF×°ÖĆ½ųŠŠCO»¹ŌFe2O3µÄŹµŃ飬a“¦µÄĻÖĻóĪŖ__________________£»øĆ×°ÖĆÖŠĘųĒņµÄ×÷ÓĆŹĒ_______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĖłŹ¾ŹĒŹµŃéŹŅ³£ÓƵÄĘųĢåÖĘČ”×°ÖĆ£®ŌŚÓĆĖ«ŃõĖ®ŗĶ¶žŃõ»ÆĆĢÖĘČ”ŃõĘųµÄŹµĮ³ÖŠ£¬æÉŃ”ÓƵķ¢ÉśŗĶŹÕ¼Æ×°ÖĆŹĒ £ØĢī×°ÖƵıąŗÅ£©£®ŌŚ“Ė·“Ó¦ÖŠ£¬¶žŃõ»ÆĆĢµÄ×÷ÓĆŹĒ £®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
»ÆѧŹµŃéŹĒѧĻ°»ÆѧµÄ»ł“”£¬Ēėøł¾ŻČēĶ¼»Ų“šĪŹĢā£ŗ
£Ø1£©ČēĶ¼¼×ŹĒŹµŃéŹŅÖĘČ”ŃõĘųµÄ×°ÖĆ£¬ÓĆĄ“¼ÓČȵÄŅĒĘ÷Ćū³ĘŹĒ £¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ £»ĪŖ·ĄÖ¹Ė®µ¹Į÷£¬ŹµŃé½įŹųŹ±Ó¦½ųŠŠµÄ²Ł×÷ĪŖ £¬Ķ¼ÖŠµÄŹÕ¼Æ×°ÖĆ»¹æÉŅŌŹÕ¼ÆµÄĘųĢåÓŠ £ØĢīŠ“Ņ»ÖÖ£©£®
£Ø2£©ČēĶ¼ŅŅ½«“ų»šŠĒµÄľĢõÉģµ½Ź¢ÓŠŃõĘųµÄ¼ÆĘųĘæÖŠ£¬æɹŪ²ģµ½µÄĻÖĻóŹĒ £¬ĖµĆ÷ŃõĘų £®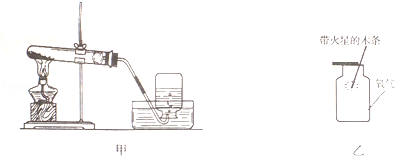
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com