
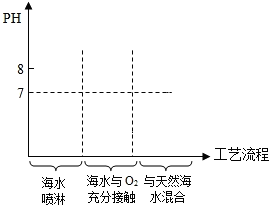
 Ϊ�˼��������Σ�����ҹ��з��ˡ���ˮ�����������ա����乤�����̴����ǣ�
Ϊ�˼��������Σ�����ҹ��з��ˡ���ˮ�����������ա����乤�����̴����ǣ����� ��1�������������pHֵ�Ĺ�ϵ���DZ��⣻
��2�����ݴӸߴ������µĺ�ˮ��pH=8.1-8.3��˵�������������ӣ�
��3����������������ñ����ӣ�
��� �⣺��1���ɺ�ˮ��Ϊ�����Լ�PHֵԽ��ԽС���ٽ��ȫ�����к�ˮ��pH���α仯��PHֵ�ֿ�ʼ��Ϳ��������𰸣�
��2����ˮ��pH=8.1-8.3��˵���Լ��ԣ����������������ӣ�
��3���ⶨ��ˮ��Na2SO4������������Ҫ֪����ˮ���������ٸ������Ȼ�����Ӧ���ɳ�����������������Ƶ�������
�𰸣�
��1��
��2������������
��3�����⺣ˮ������BaCl2��
���� ͨ���ش���֪����pHֵ���������Ĺ�ϵ���˽��˲ⶨ���������Ȼ�����ʵ��Ĺ��̣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | AgCl ����ɫ�� | Ag2S ����ɫ�� | CaSO4 ����ɫ�� | Ca��OH��2 ����ɫ�� | Ca��HCO3��2 ����ɫ�� | CaCO3 ����ɫ�� | NH4HCO3 ��ɫ�� | ��NH4��2SO4 ��ɫ�� |
| �ܽ� ��/g | 1.5��10-4 | 1.3��10-6 | 0.2 | 0.165 | 16.6 | 0.0015 | 21 | 75.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 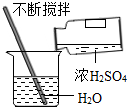 ϡ��Ũ���� | B�� | 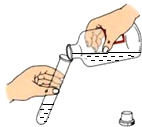 �㵹Һ�� | C�� | 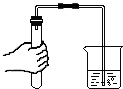 ��������� | D�� | 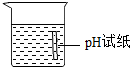 �ⶨ��ҺpH �ⶨ��ҺpH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
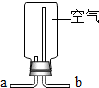 | 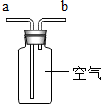 | 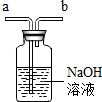 | 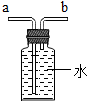 |
| ���ſ������ռ�CO2����CO2��b��ͨ�� | ���ſ������ռ�O2����O2��a��ͨ�� | ��ȥ�����е�CO2���������a��ͨ�� | ����ˮ���ռ�O2����O2��b��ͨ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ͼ��NaCl��MgSO4�������ʵ��ܽ�����ߣ����ܽ�����ߵó����¼�����Ϣ��������ȷ���ǣ�������
��ͼ��NaCl��MgSO4�������ʵ��ܽ�����ߣ����ܽ�����ߵó����¼�����Ϣ��������ȷ���ǣ�������| A�� | NaCl���ܽ�ȴ���MgSO4���ܽ�� | |
| B�� | 0��ʱ��NaCl��MgSO4�������ʵ��ܽ�ȶ�Ϊ0�� | |
| C�� | t1��ʱ��NaCl��MgSO4�ı�����Һ����������������� | |
| D�� | ��MgSO4�ı�����Һ��t3�潵��t2�棬���о������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ʵ��������MnO2��ΪKClO3�ֽ��������Ĵ�������ѧ����ʽΪ��
��ʵ��������MnO2��ΪKClO3�ֽ��������Ĵ�������ѧ����ʽΪ��| ʵ�� ��� | KClO3�������ˣ� | ���� | �������� �������ˣ� | ��ʱ ���룩 | |
| ��ѧʽ | �������ˣ� | ||||
| 1 | 0.60 | - | - | 0.014 | 480 |
| 2 | 0.60 | MnO2 | 0.20 | 0.096 | 36.5 |
| 3 | 0.60 | CuO | 0.20 | 0.096 | 79.5 |
| 4 | 0.60 | Fe2O3 | 0.20 | 0.096 | 34.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
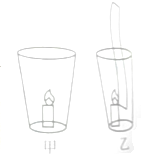 ��ͼ����ʾ�����е�����ȼ��һ��ʱ�佫Ϩ������һ��ֽ���뱭�У���ͼ����ʾ����������һֱȼ����ȥ���������ԭ��
��ͼ����ʾ�����е�����ȼ��һ��ʱ�佫Ϩ������һ��ֽ���뱭�У���ͼ����ʾ����������һֱȼ����ȥ���������ԭ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��-�٢� | B�� | ������-�ۢܢ� | C�� | �����-�ڢ� | D�� | ��-�٢� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com